Electron Configurations Quantum Mechanical Model of the Atom






- Slides: 21

Electron Configurations

Quantum Mechanical Model of the Atom • The Quantum Mechanical Model is the current description of electrons in atoms. - does not describe the electron’s path around the nucleus • The Quantum Mechanical Model is based on several ideas including: –Schrodinger wave equation (1926) treats electrons as waves. –Heisenberg uncertainty principle (1927) states that it is impossible to know both the velocity and position of a particle at the same time.


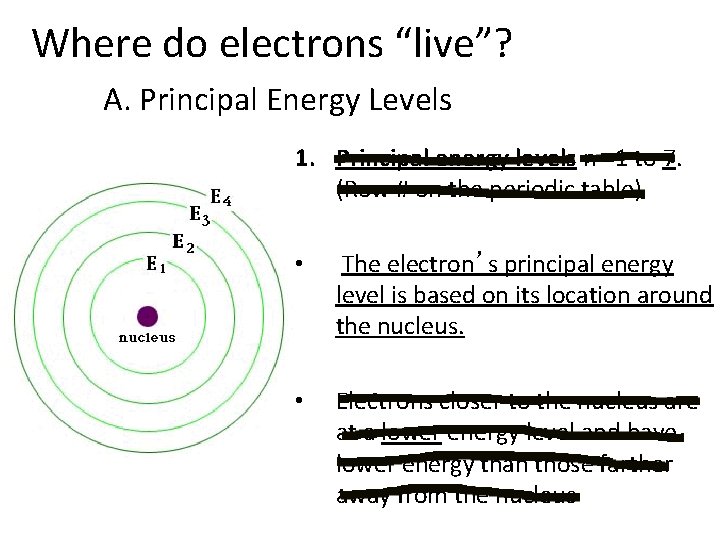
Where do electrons “live”? A. Principal Energy Levels 1. Principal energy levels n =1 to 7. (Row # on the periodic table) • The electron’s principal energy level is based on its location around the nucleus. • Electrons closer to the nucleus are at a lower energy level and have lower energy than those farther away from the nucleus

Energy levels are like rungs of a ladder. You cannot be in between a rung Energy levels in an atom’s electron are unequally spaced. The higher energy levels are closer together.

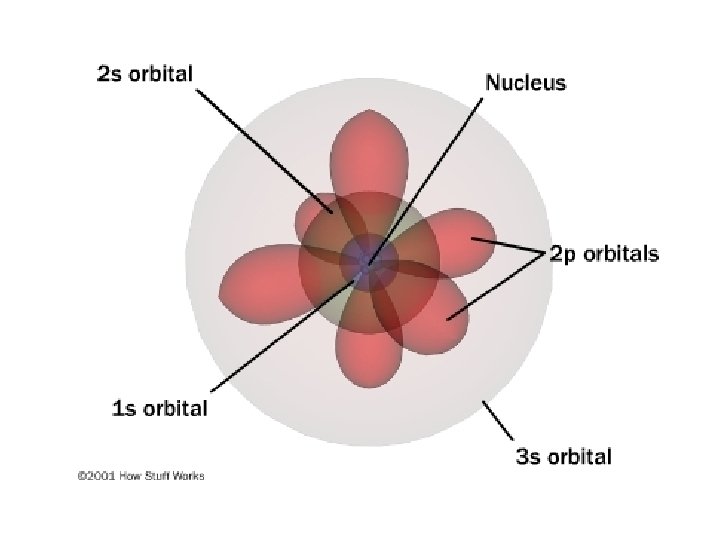
B. Atomic Orbitals • An atomic orbital is a region of space in which there is a high probability of finding an electron • assigned letters s, p, d or f (smart people do fine) • Energy sublevels correspond to a shape where the electron is likely to be found.

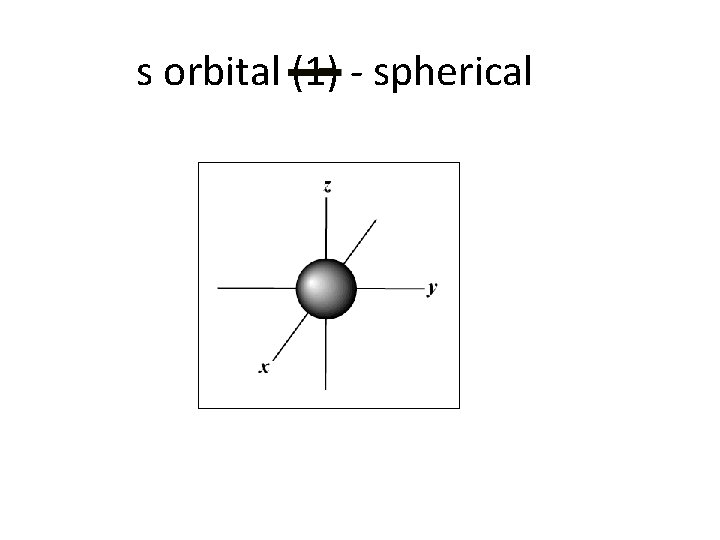
s orbital (1) - spherical

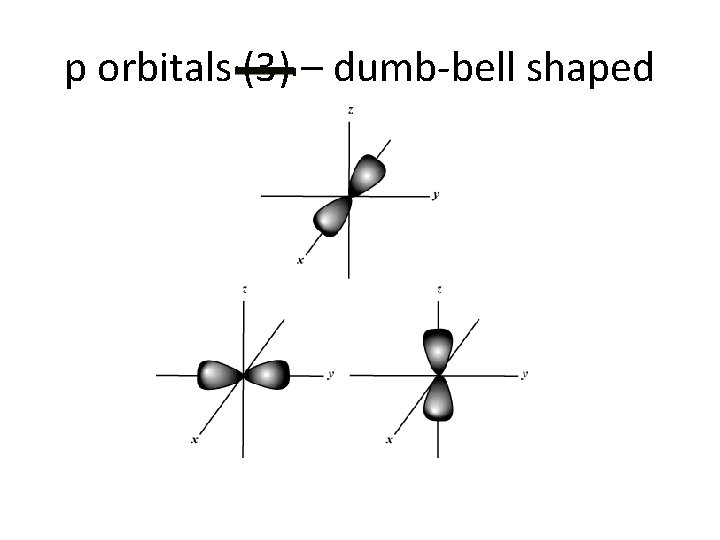
p orbitals (3) – dumb-bell shaped

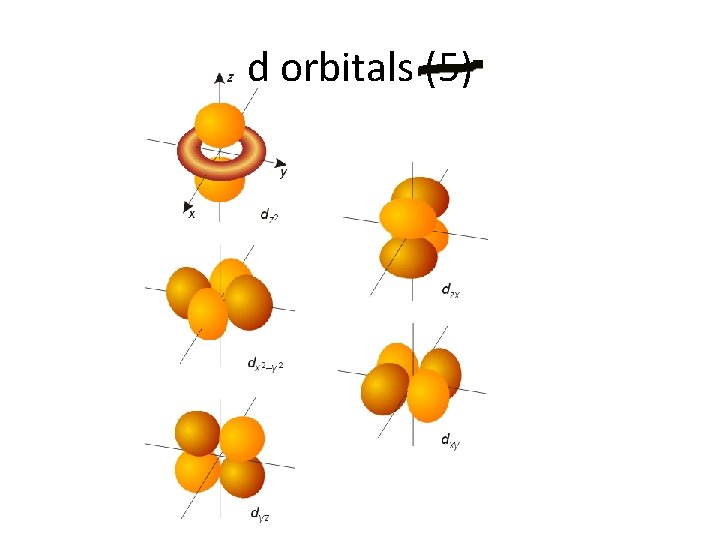
d orbitals (5)

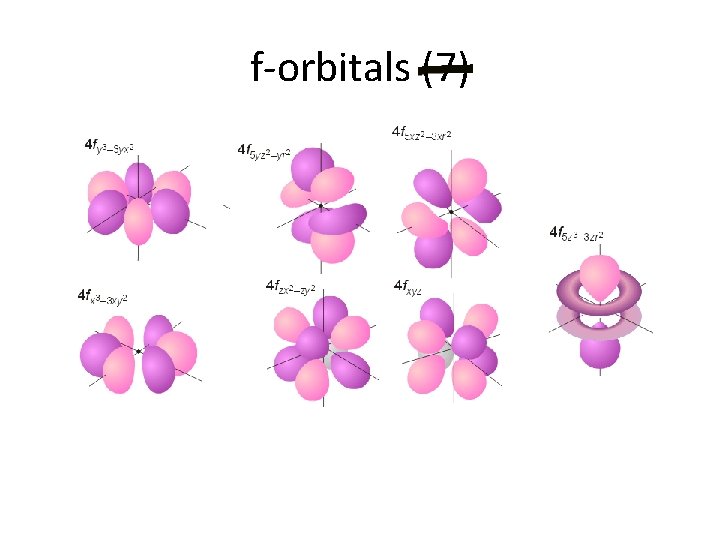
f-orbitals (7)

Energy Levels, Sublevels, and Orbitals 1. Principal energy levels – n, assigned values 1 -7 (Like floors in a hotel) 2. Energy sublevels- s, p, d, f (Type of suite in a hotel) § § s sublevel – 1 orbital p sublevel – 3 orbitals d sublevel – 5 orbitals f sublevel – 7 orbitals (Orbitals are like the number of rooms in a suite) 3. Orbitals – Two electrons per orbital (Two people per room)

Electron Configurations • Electron configuration – the arrangement of electrons in an atom. • Example Sodium (Na) – 1 s 22 p 63 s 1 • Three rules determine electron configurations – the Aufbau Principle, – the Pauli Exclusion Principle – Hund’s rule

The Aufbau Principle • Each electron occupies the lowest energy orbital available • Like filling the hotel from the bottom up

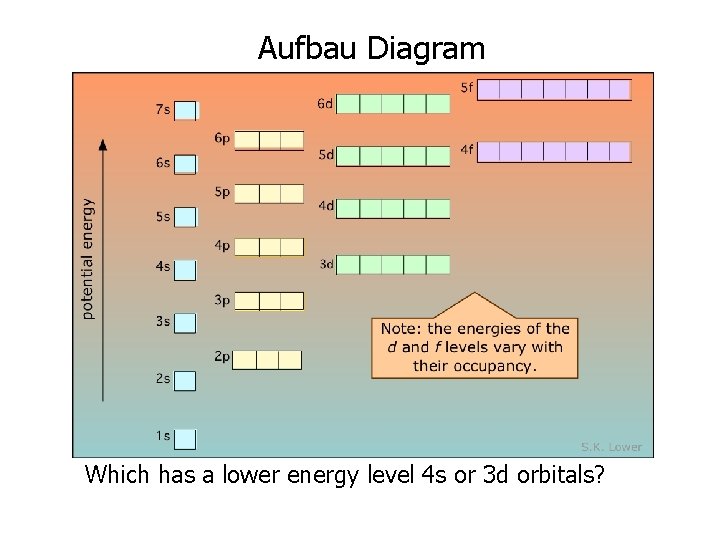
Aufbau Diagram Which has a lower energy level 4 s or 3 d orbitals?

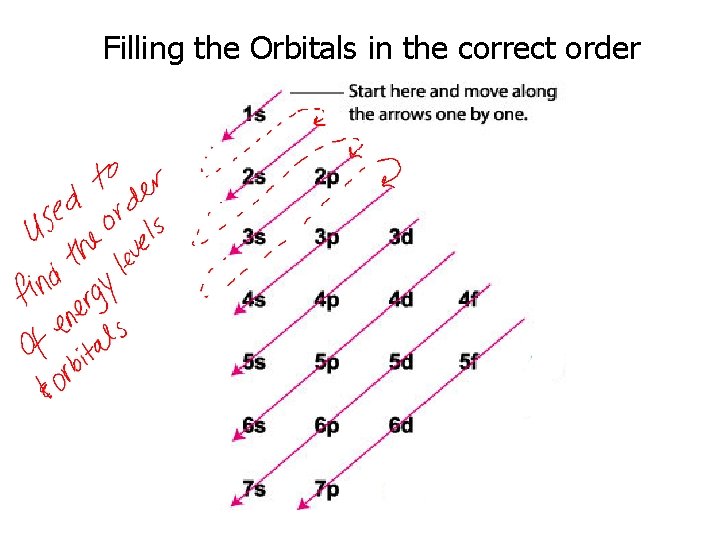
Filling the Orbitals in the correct order

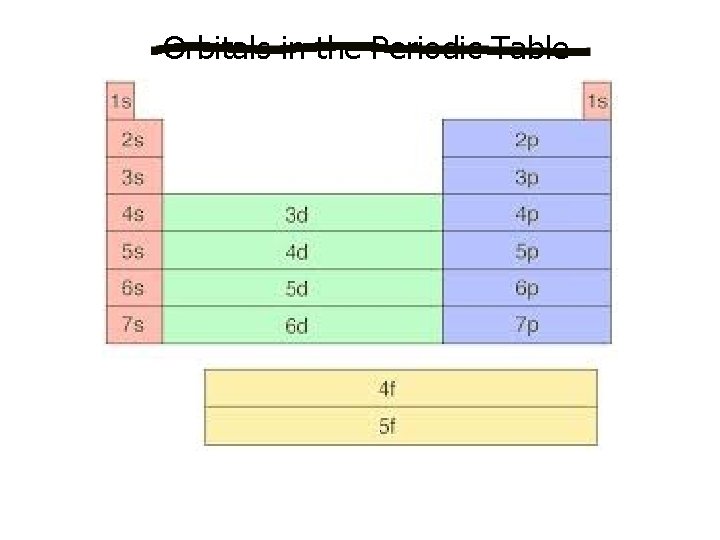
Orbitals in the Periodic Table

Pauli Exclusion Principle • A maximum of two electrons may occupy a single orbital • Like only two people sharing one hotel room

Hund’s Rule • If two or more orbitals of equal energy are available, electrons will occupy them singly with the same spin, before filling them in pairs with opposite spins • A spin is denoted with an up or down arrow to fill orbitals • This is like trying to find your own room in the same suite before having to share a room with someone else

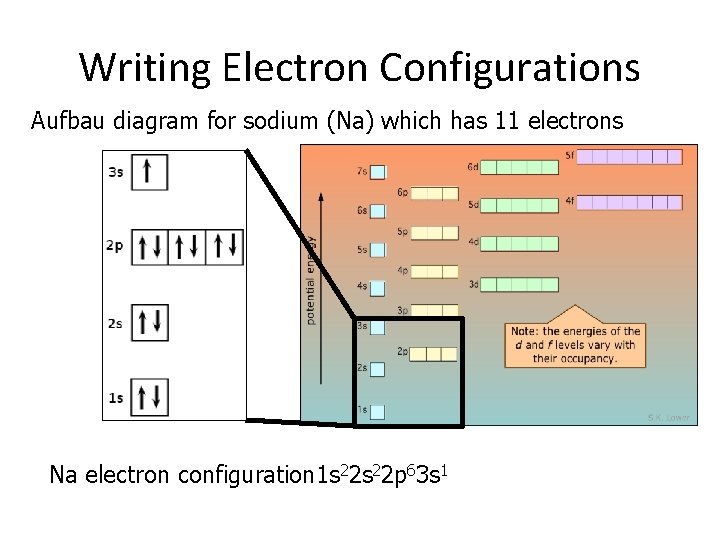
Writing Electron Configurations Aufbau diagram for sodium (Na) which has 11 electrons Na electron configuration 1 s 22 p 63 s 1

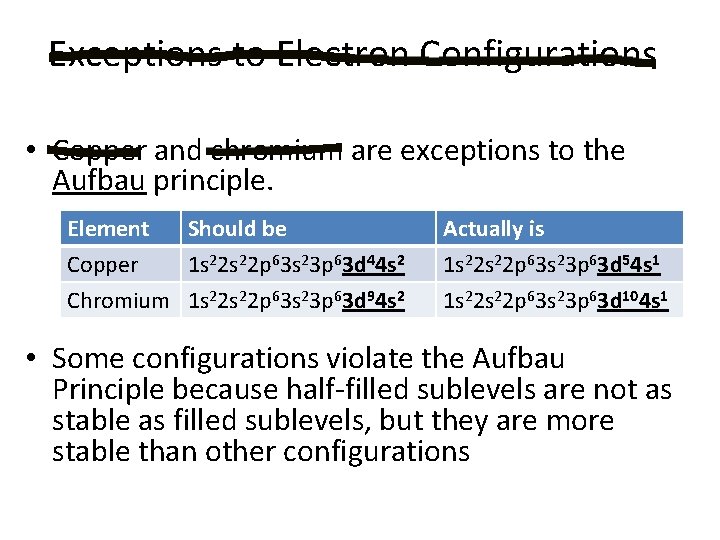
Exceptions to Electron Configurations • Copper and chromium are exceptions to the Aufbau principle. Element Should be Copper 1 s 22 p 63 s 23 p 63 d 44 s 2 Chromium 1 s 22 p 63 s 23 p 63 d 94 s 2 Actually is 1 s 22 p 63 s 23 p 63 d 54 s 1 1 s 22 p 63 s 23 p 63 d 104 s 1 • Some configurations violate the Aufbau Principle because half-filled sublevels are not as stable as filled sublevels, but they are more stable than other configurations

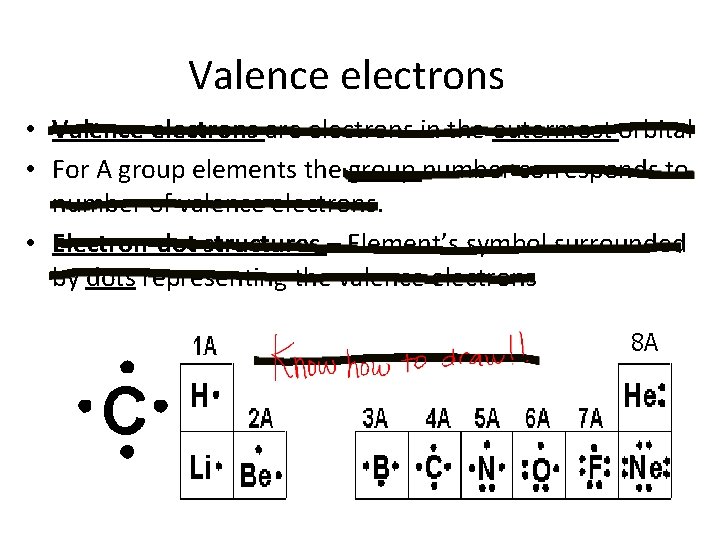
Valence electrons • Valence electrons are electrons in the outermost orbital • For A group elements the group number corresponds to number of valence electrons. • Electron-dot structures – Element’s symbol surrounded by dots representing the valence electrons 8 A