Electron Configurations of Atoms Electron Configurations Electron Configurations



- Slides: 16

Electron Configurations of Atoms

Electron Configurations • Electron Configurations – the way electrons are arranged in atomic orbitals. This describes the location and energy of the electrons. • To do this you need to know the relative energy levels of the orbitals • Note: Have your periodic table in hand

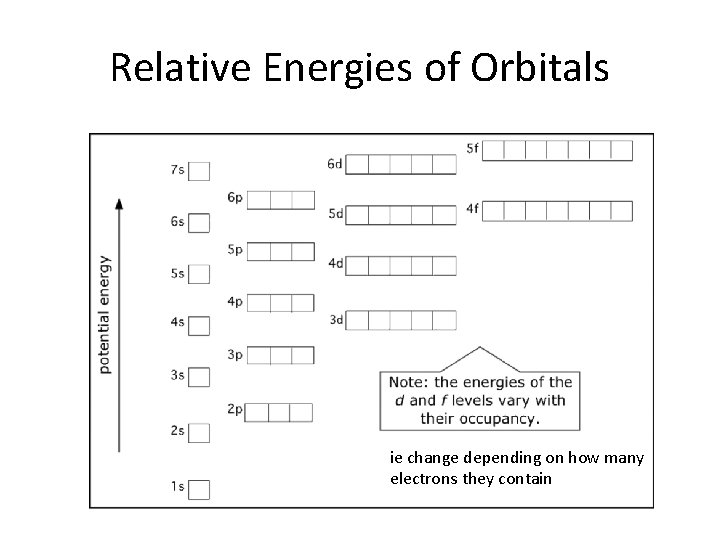
Relative Energies of Orbitals ie change depending on how many electrons they contain

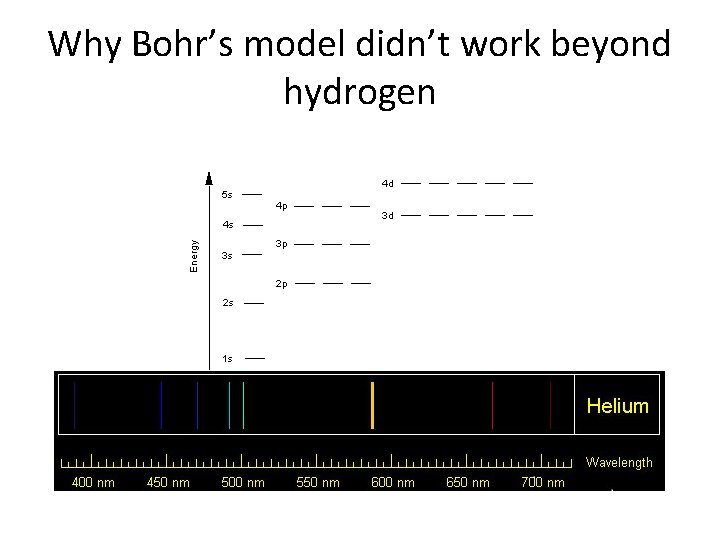
Relative Orbital Energies • Notice that within each principal energy level the order is s, p, d, f • Above 3 p however there is overlap between different principal energy levels – e. g. 4 s is lower than 3 d • You will see later that this can be determined by the structure of the periodic table

Rules for Determining Configuration 1. Aufbau Principle: Lower energy orbitals are filled first one electron at a time 2. Pauli Exclusion Principle: only two electrons in one orbital and they must have opposite spin 3. Hund’s Rule: Equal energy orbitals must be filled with unpaired electrons before paired ones

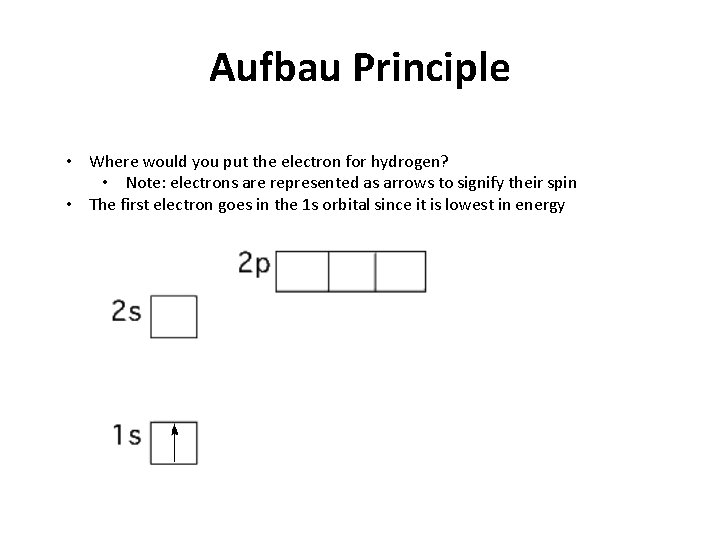
Aufbau Principle • Where would you put the electron for hydrogen? • Note: electrons are represented as arrows to signify their spin • The first electron goes in the 1 s orbital since it is lowest in energy

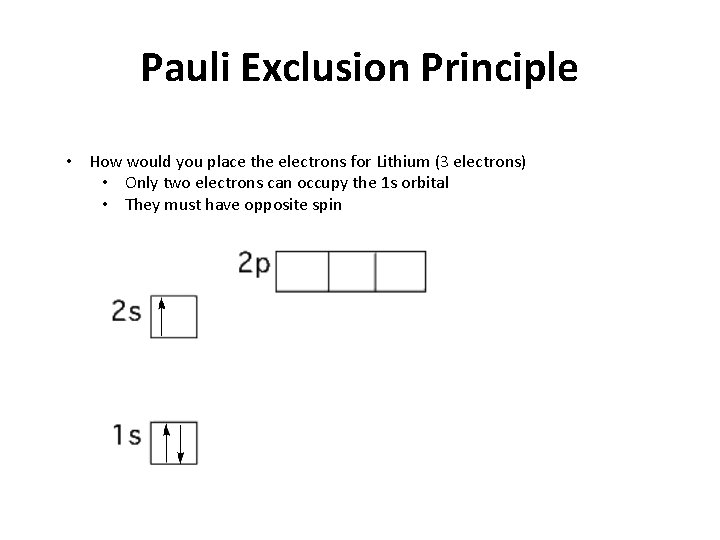
Pauli Exclusion Principle • How would you place the electrons for Lithium (3 electrons) • Only two electrons can occupy the 1 s orbital • They must have opposite spin

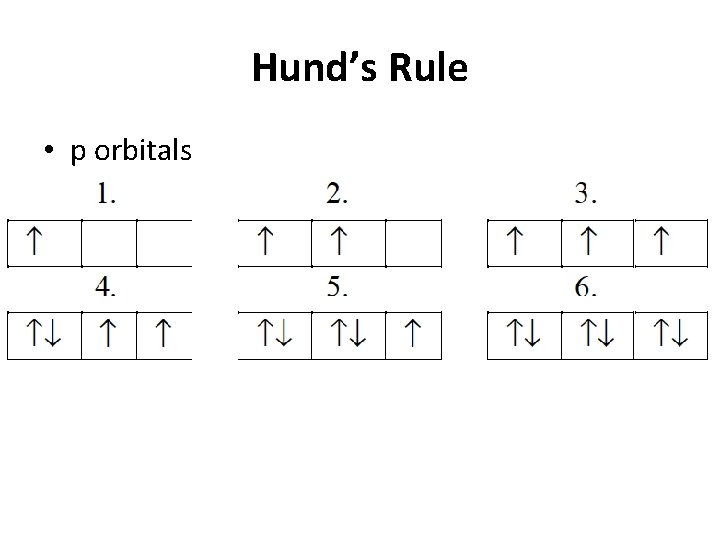
Hund’s Rule • p orbitals

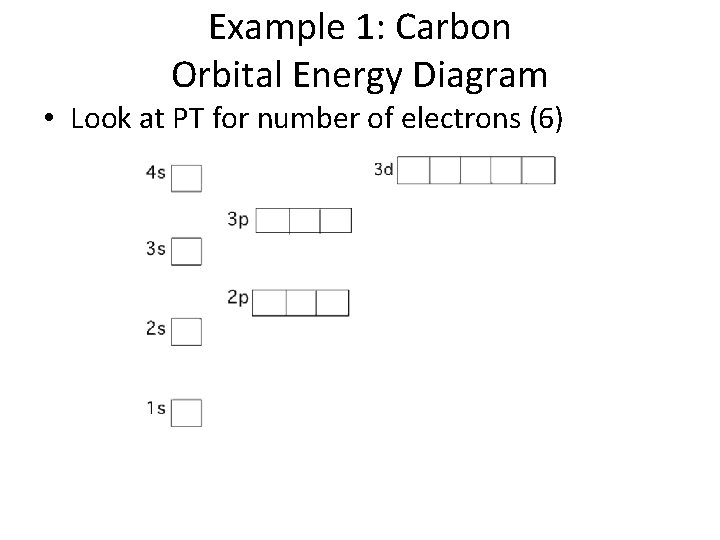
Example 1: Carbon Orbital Energy Diagram • Look at PT for number of electrons (6)

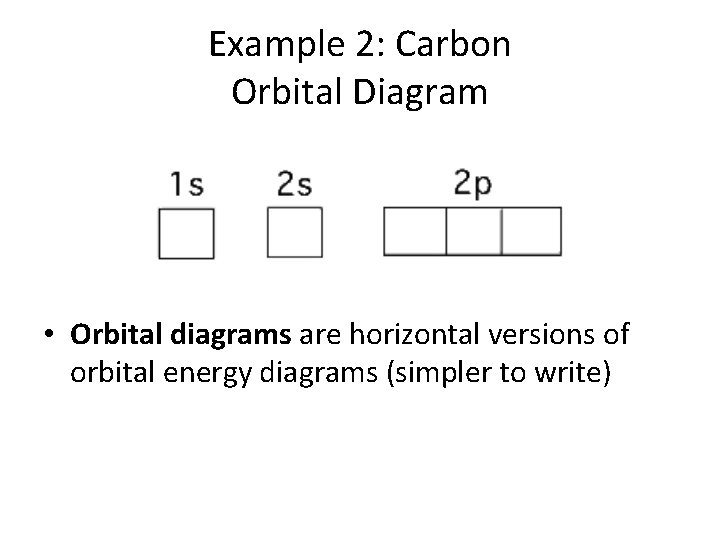
Example 2: Carbon Orbital Diagram • Orbital diagrams are horizontal versions of orbital energy diagrams (simpler to write)

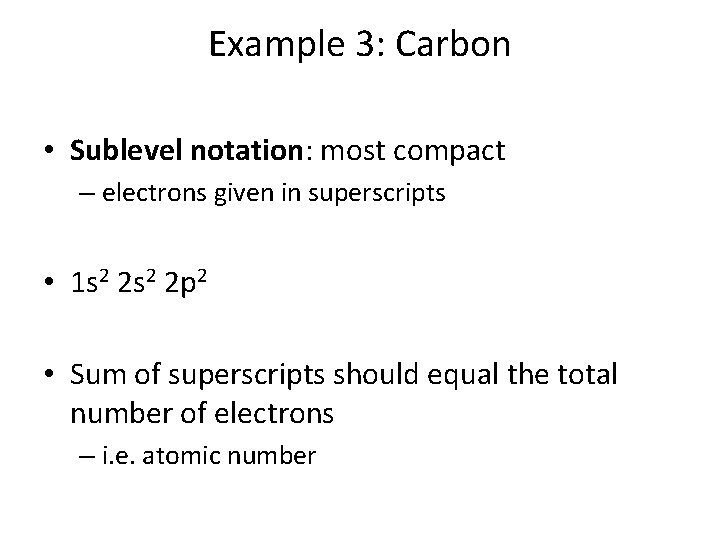
Example 3: Carbon • Sublevel notation: most compact – electrons given in superscripts • 1 s 2 2 p 2 • Sum of superscripts should equal the total number of electrons – i. e. atomic number

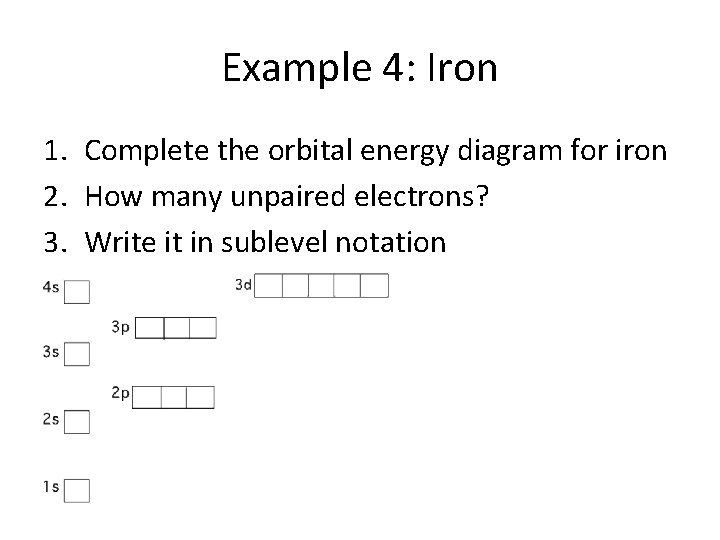
Example 4: Iron 1. Complete the orbital energy diagram for iron 2. How many unpaired electrons? 3. Write it in sublevel notation

Why Bohr’s model didn’t work beyond hydrogen

Example 5: Chloride anion Cl • Write in sublevel notation: • 1 s 2 2 p 6 3 s 2 3 p 6 • Note: it has the same electron configuration as Argon

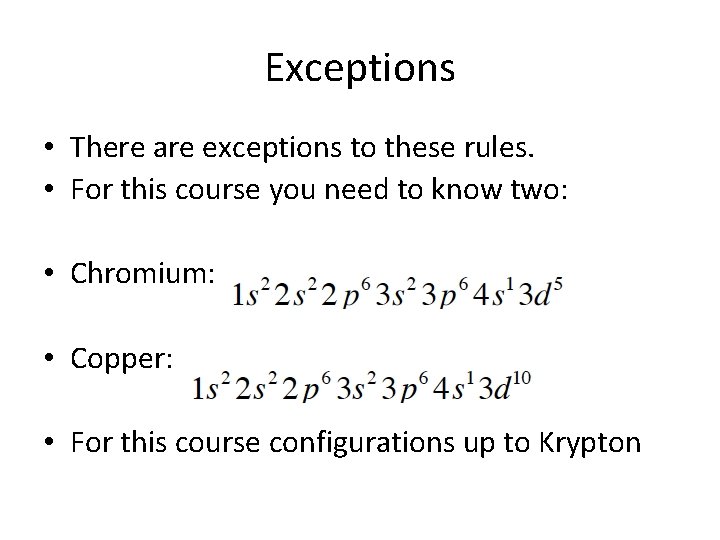
Exceptions • There are exceptions to these rules. • For this course you need to know two: • Chromium: • Copper: • For this course configurations up to Krypton

Worksheet