Electron Configurations Each element has a unique electron

![Electron Configurations Shorthand Configuration: [Noble Gas] __s 2 __p 6 etc -Use the noble Electron Configurations Shorthand Configuration: [Noble Gas] __s 2 __p 6 etc -Use the noble](https://slidetodoc.com/presentation_image_h2/41dcbc16395f922570c6474cd37225ed/image-8.jpg)
- Slides: 9

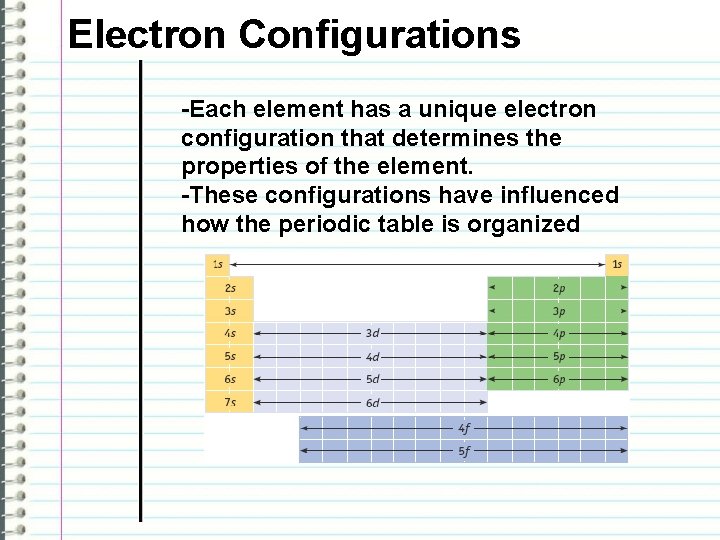
Electron Configurations -Each element has a unique electron configuration that determines the properties of the element. -These configurations have influenced how the periodic table is organized

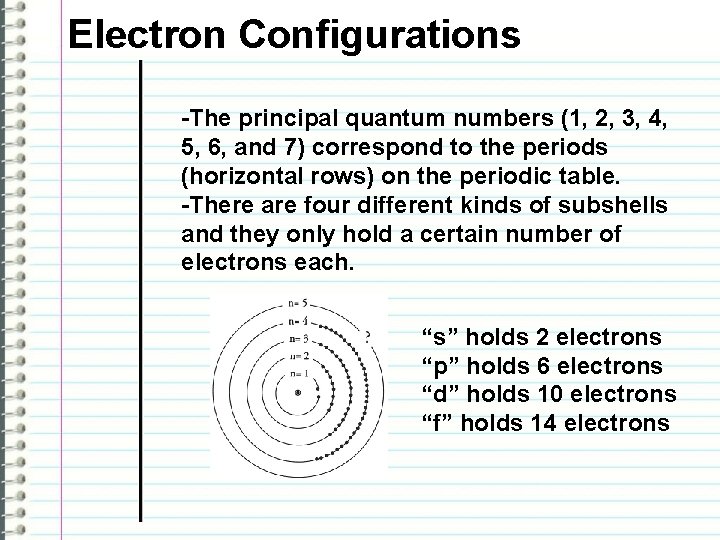
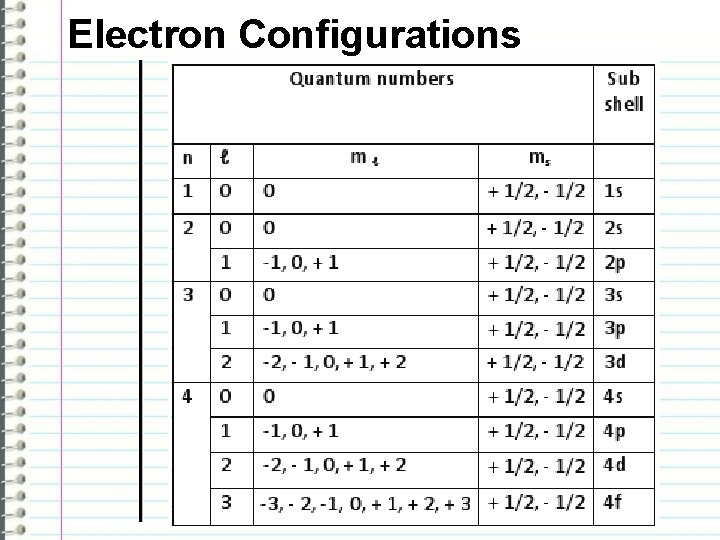
Electron Configurations -The principal quantum numbers (1, 2, 3, 4, 5, 6, and 7) correspond to the periods (horizontal rows) on the periodic table. -There are four different kinds of subshells and they only hold a certain number of electrons each. “s” holds 2 electrons “p” holds 6 electrons “d” holds 10 electrons “f” holds 14 electrons

Electron Configurations The order of the subshells and quantum numbers: 1 s 2 s 2 p 3 s 3 p 4 s 3 d 4 p 5 s 4 d 5 p 6 s 4 f 5 d 6 p 7 s 5 f 6 d -Electron configurations determine how many valence electrons an element will have based on the highest level orbitals -Patterns in valence electrons determine chemical reactivity

Electron Configurations

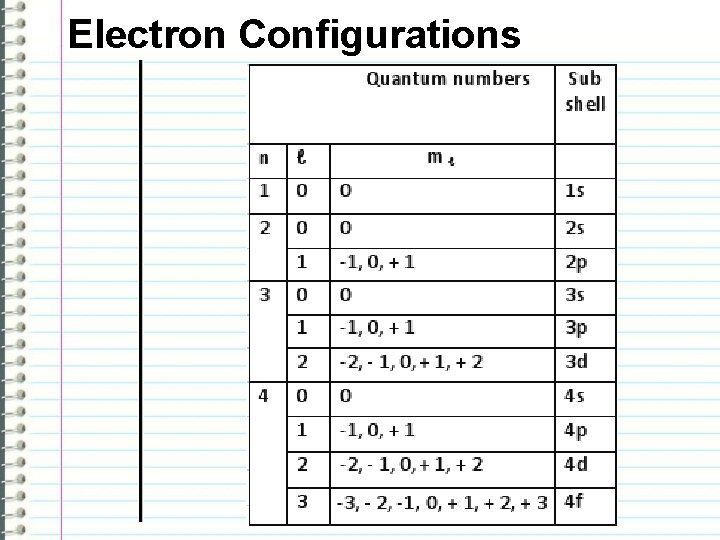
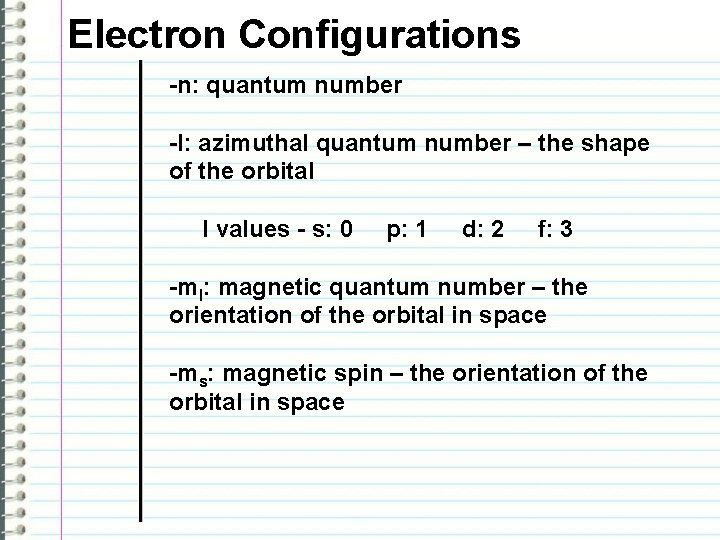
Electron Configurations -n: quantum number -l: azimuthal quantum number – the shape of the orbital l values - s: 0 p: 1 d: 2 f: 3 -ml: magnetic quantum number – the orientation of the orbital in space -ms: magnetic spin – the orientation of the orbital in space

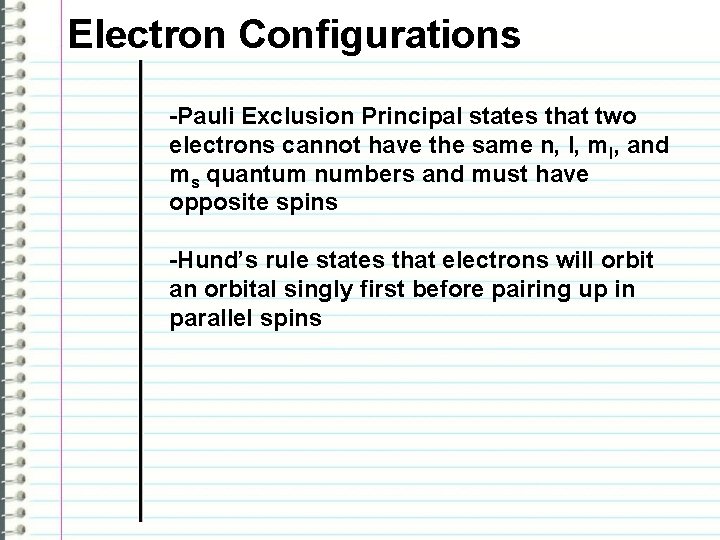
Electron Configurations -Pauli Exclusion Principal states that two electrons cannot have the same n, l, ml, and ms quantum numbers and must have opposite spins -Hund’s rule states that electrons will orbit an orbital singly first before pairing up in parallel spins

Electron Configurations
![Electron Configurations Shorthand Configuration Noble Gas s 2 p 6 etc Use the noble Electron Configurations Shorthand Configuration: [Noble Gas] __s 2 __p 6 etc -Use the noble](https://slidetodoc.com/presentation_image_h2/41dcbc16395f922570c6474cd37225ed/image-8.jpg)
Electron Configurations Shorthand Configuration: [Noble Gas] __s 2 __p 6 etc -Use the noble gas located periodically before the element you’re looking at and write the rest that follows Examples: Na: [Ne] 3 s 1 Se: [Ar] 4 s 2 3 d 10 4 p 4

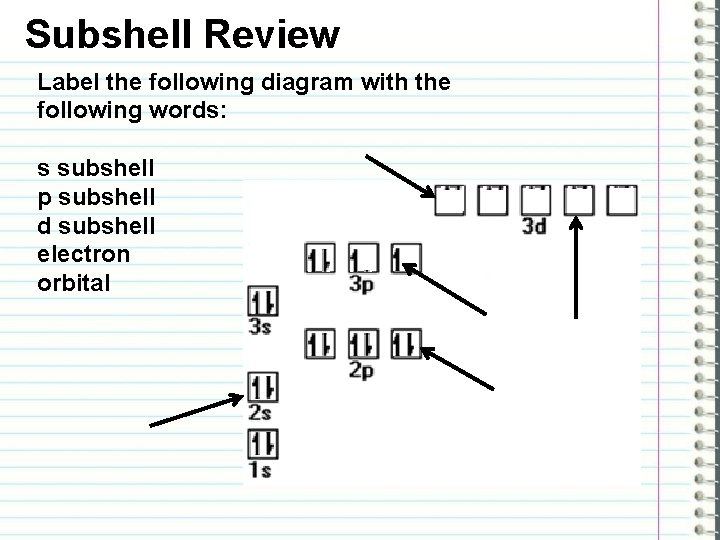
Subshell Review Label the following diagram with the following words: s subshell p subshell d subshell electron orbital