Electron Configurations and the Aufbau Principle Mr Tsigaridis
Electron Configurations and the Aufbau Principle Mr. Tsigaridis
The Periodic Table When creating electron configurations we must first take a look at how the periodic table of the elements is separated based on orbitals of atoms There are 4 major orbitals that we will be discussing, with a focus on the first three
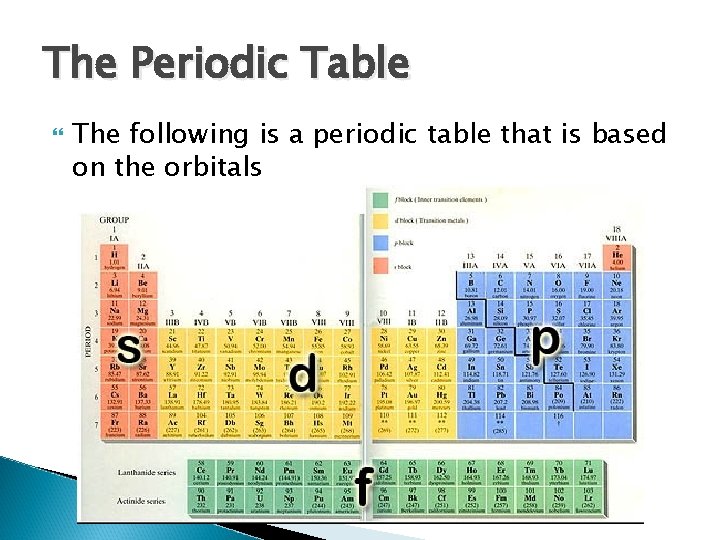
The Periodic Table The following is a periodic table that is based on the orbitals

Filling the Orbitals When filling the orbitals with electrons, we must fill the orbitals with the lowest energy first Not only do the orbitals with the lowest energy have to be filled first, but they also must become half filled with electrons with one type of spin first in order to follow the Pauli exclusion principle
Filling the Orbitals There are two ways to show electron configurations The first way is pictorially using boxes and arrows and the second way is the condensed electron configurations Before continuing with the electron configurations, we must discuss the order in increasing energy of the orbitals in question
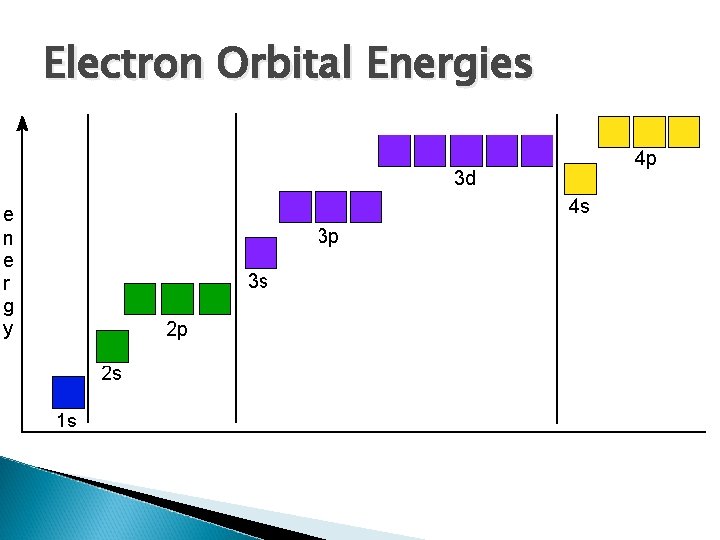
Electron Orbital Energies

“ 3 d” and “ 4 s” Orbitals You may have noticed that the 3 d orbitals are at a higher energy than the 4 s obritals To understand why this happens we have to go back to some of the fundamentals of quantum chemistry Electrons are attracted to the nucleus and so to pull an electron farther away from the nucleus, you have to work against this attraction That gives rise to the idea that electrons that are further out have higher energies

“ 3 d” and “ 4 s” Orbitals For atoms that are heavier than copper this effect dominates and the 4 s orbitals have a higher energy than the 3 d electrons Raising the energy however has another effect and that is the fact that you get more nuclear nodes Those areas where you have a 0 probability of finding electrons close to the nucleus
“ 3 d” and “ 4 s” Orbitals This gives higher energy orbitals (like d orbitals) more energy than the lower energy orbitals like the s orbitals As the orbitals fill, with more unpaired spins, it means a lower energy overall This causes the 4 s orbitals and the 3 d orbitals to equalize in terms of the energy of the electrons in each orbital
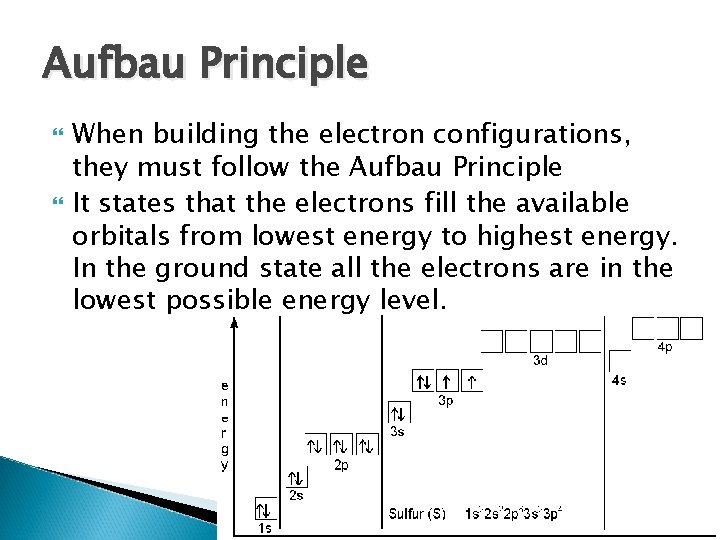
Aufbau Principle When building the electron configurations, they must follow the Aufbau Principle It states that the electrons fill the available orbitals from lowest energy to highest energy. In the ground state all the electrons are in the lowest possible energy level.
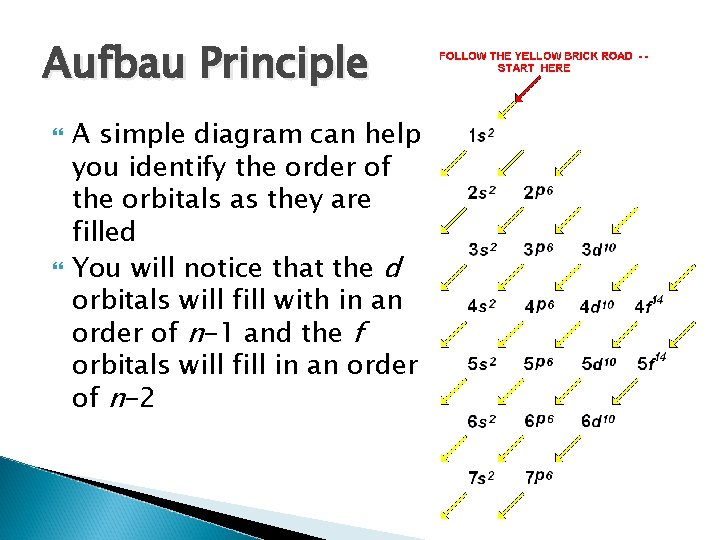
Aufbau Principle A simple diagram can help you identify the order of the orbitals as they are filled You will notice that the d orbitals will fill with in an order of n-1 and the f orbitals will fill in an order of n-2
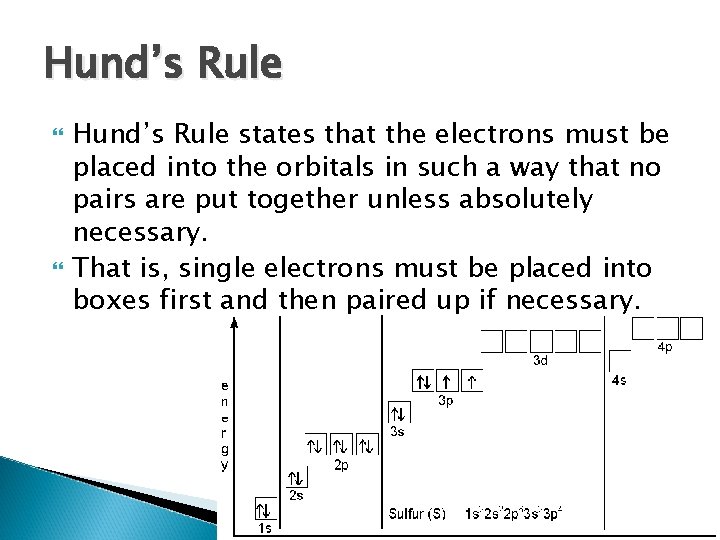
Hund’s Rule states that the electrons must be placed into the orbitals in such a way that no pairs are put together unless absolutely necessary. That is, single electrons must be placed into boxes first and then paired up if necessary.
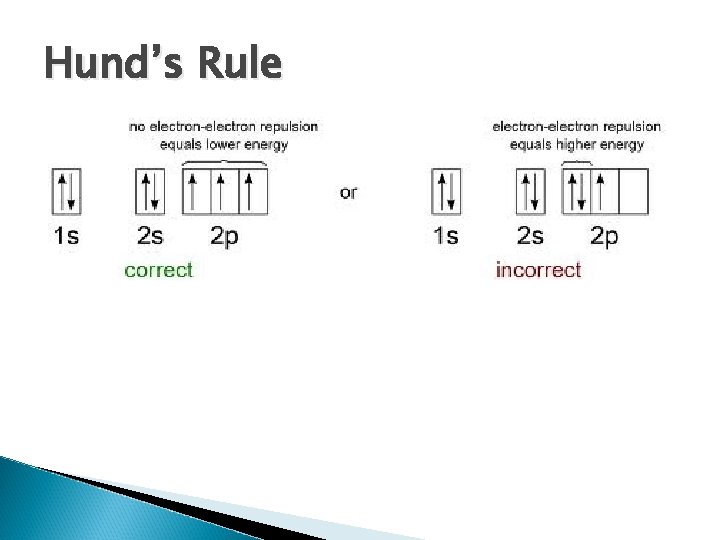
Hund’s Rule
- Slides: 13