Electron Configurations and Periodicity Why is this VERY







- Slides: 14

Electron Configurations and Periodicity. Why is this VERY important? y Chemistry is explained through sharing or transferring electrons. y This determines which elements can bond and what type of bond they form

How are the electrons arranged in an atom? What orbitals are preferentially filled by electrons? y What is an electron configuration?

Energy levels and sublevels y Energy levels (refer to periods on periodic table) y Also referred to as “shells” y 7 energy levels 1, 2, 3, 4 … 7 y Energy level 1 is lowest in energy and closest to the nucleus C. Johannesson

Sub-levels y The main energy levels contain sub-levels (refer to groups on periodic table #1 -18) y The different main energy levels have different sub-levels in them y There are four types based on shape of orbitals: s (groups 1 -2) lowest energy , p, (13 -18) d (transition metals), f (inner transition metals) highest energy C. Johannesson

Electrons in Atoms Electron Configuration C. Johannesson

3 RULES FOR THE ARRANGEMENT OF ELECTRONS IN ATOMS. Hints for rules z 1) The Pauli Exclusion Principle (not allowed) z 2) The Aufbau Principle (building up or Lazy tenant rule) z 3) Hund’s Rule (empty bus seat rule) C. Johannesson

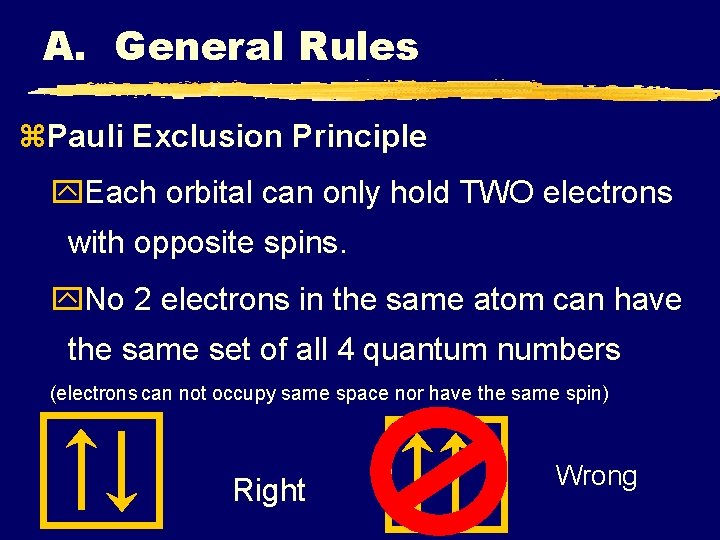
A. General Rules z. Pauli Exclusion Principle y. Each orbital can only hold TWO electrons with opposite spins. y. No 2 electrons in the same atom can have the same set of all 4 quantum numbers (electrons can not occupy same space nor have the same spin) Right Wrong

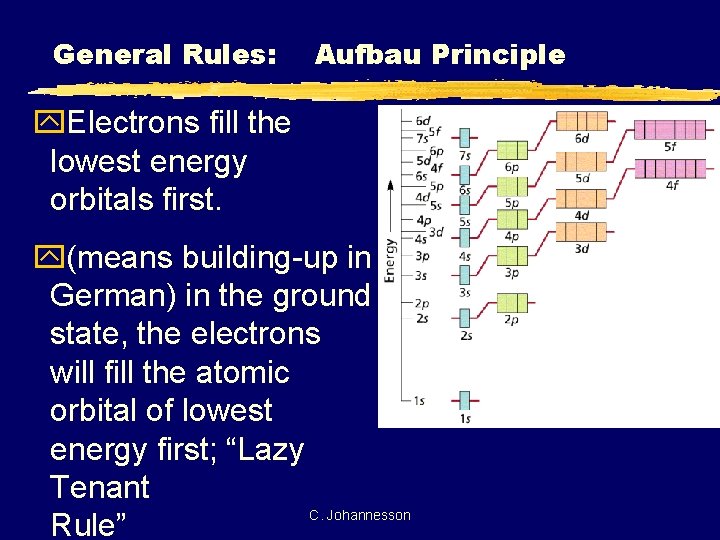
General Rules: Aufbau Principle y. Electrons fill the lowest energy orbitals first. y(means building-up in German) in the ground state, the electrons will fill the atomic orbital of lowest energy first; “Lazy Tenant C. Johannesson Rule”

A. General Rules z. Hund’s Rule y. Within a sublevel, place one electron per orbital before pairing them. y“Empty Bus Seat Rule” WRONG C. Johannesson RIGHT

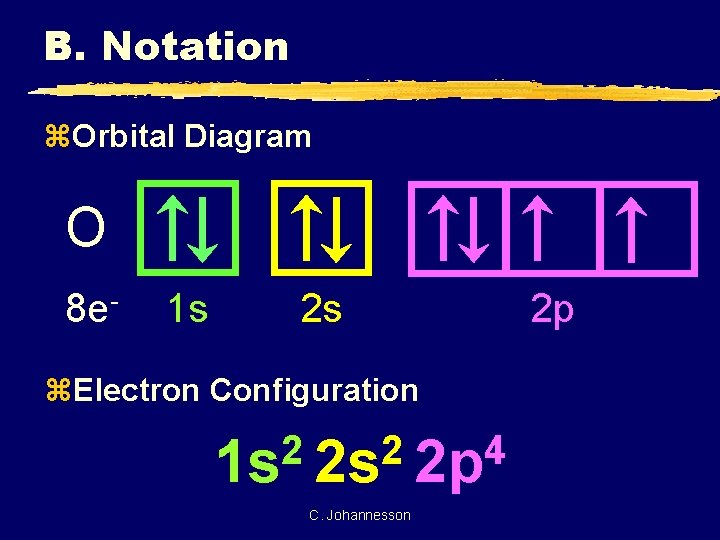
B. Notation z. Orbital Diagram O 8 e- 1 s 2 s z. Electron Configuration 2 2 4 1 s 2 s 2 p C. Johannesson 2 p

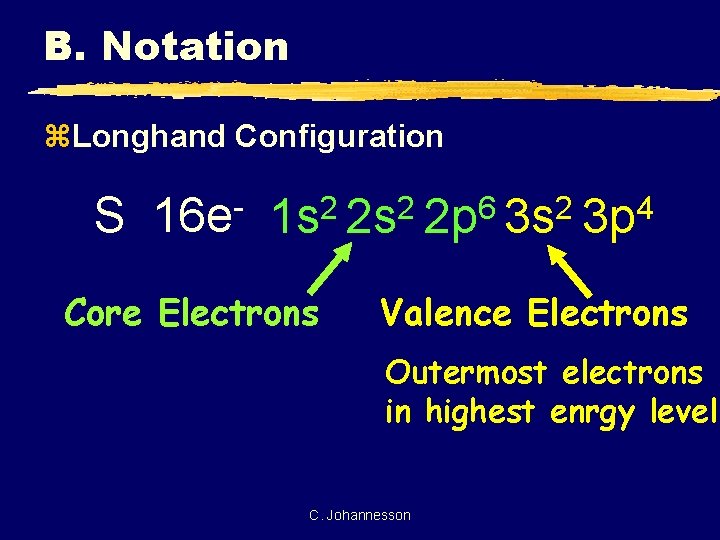
B. Notation z. Longhand Configuration S 16 e 6 2 2 2 1 s 2 s 2 p 3 s Core Electrons 4 3 p Valence Electrons Outermost electrons in highest enrgy level C. Johannesson

C. Periodic Patterns s p 1 2 3 4 5 6 7 f (n-2) d (n-1) 6 7 © 1998 by Harcourt Brace & Company C. Johannesson

C. Periodic Patterns z. Period # yenergy level (subtract for d & f) z. Group # ytotal # of valence ez. Column within sublevel block y# of e- in sublevel C. Johannesson

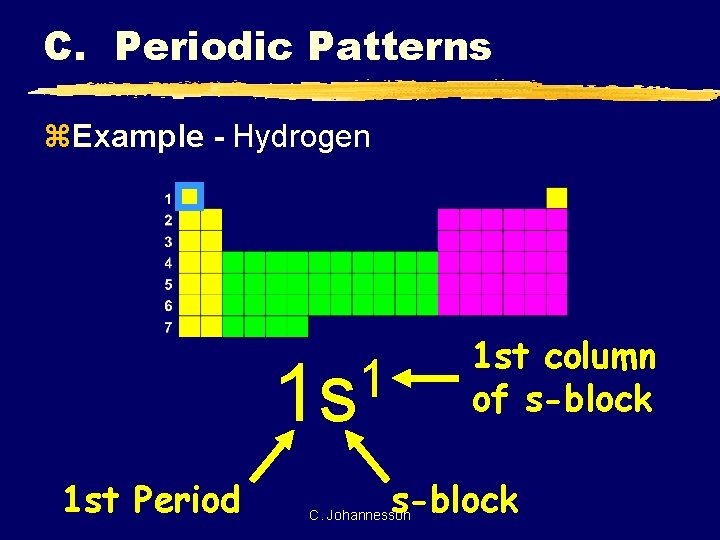
C. Periodic Patterns z. Example - Hydrogen 1 st column of s-block 1 1 s 1 st Period s-block C. Johannesson