Electron Configurations and Periodicity Why is this VERY






![C. Periodic Patterns z. Example - Germanium [Ar] 2 4 s 10 3 d C. Periodic Patterns z. Example - Germanium [Ar] 2 4 s 10 3 d](https://slidetodoc.com/presentation_image_h/df8713bd517ba0a975c9792279696946/image-14.jpg)

![D. Stability z. Electron Configuration Exceptions y. Copper EXPECT: [Ar] 4 s 2 3 D. Stability z. Electron Configuration Exceptions y. Copper EXPECT: [Ar] 4 s 2 3](https://slidetodoc.com/presentation_image_h/df8713bd517ba0a975c9792279696946/image-16.jpg)
![D. Stability z. Electron Configuration Exceptions y. Chromium EXPECT: [Ar] 4 s 2 3 D. Stability z. Electron Configuration Exceptions y. Chromium EXPECT: [Ar] 4 s 2 3](https://slidetodoc.com/presentation_image_h/df8713bd517ba0a975c9792279696946/image-17.jpg)
- Slides: 19

Electron Configurations and Periodicity. Why is this VERY important? y Chemistry is explained through sharing or transferring electrons. y This determines which elements can bond and what type of bond they form

How are the electrons arranged in an atom? What orbitals are preferentially filled by electrons? y What is an electron configuration?

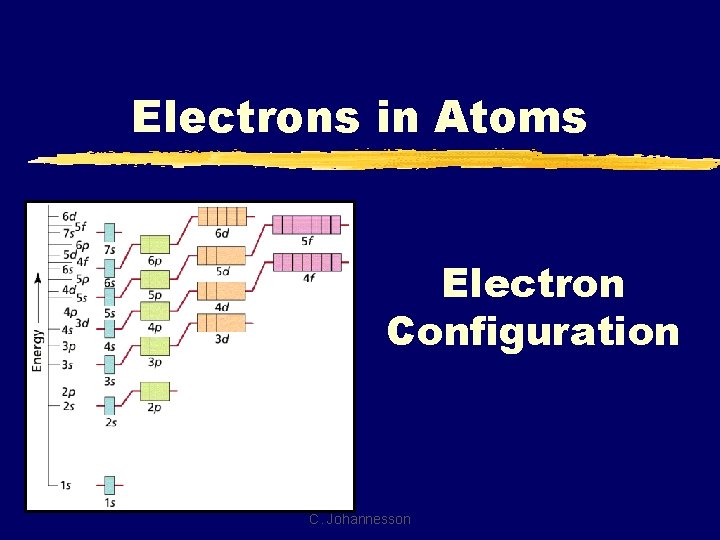
Electrons in Atoms Electron Configuration C. Johannesson

3 RULES FOR THE ARRANGEMENT OF ELECTRONS IN ATOMS. z 1) The Pauli Exclusion Principle z 2) The Aufbau Principle z 3) Hund’s Rule C. Johannesson

A. General Rules z. Pauli Exclusion Principle y. Each orbital can only hold TWO electrons with opposite spins. y. No 2 electrons in the same atom can have the same set of all 4 quantum numbers (electrons can not occupy same space nor have the same spin) Right Wrong

General Rules: Aufbau Principle y. Electrons fill the lowest energy orbitals first. y(means building-up in German) in the ground state, the electrons will fill the atomic orbital of lowest energy first; “Lazy Tenant C. Johannesson Rule”

A. General Rules z. Hund’s Rule y. Within a sublevel, place one electron per orbital before pairing them. y“Empty Bus Seat Rule” WRONG C. Johannesson RIGHT

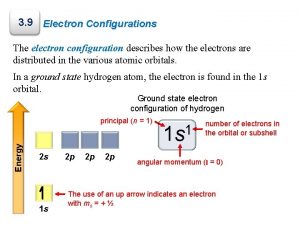
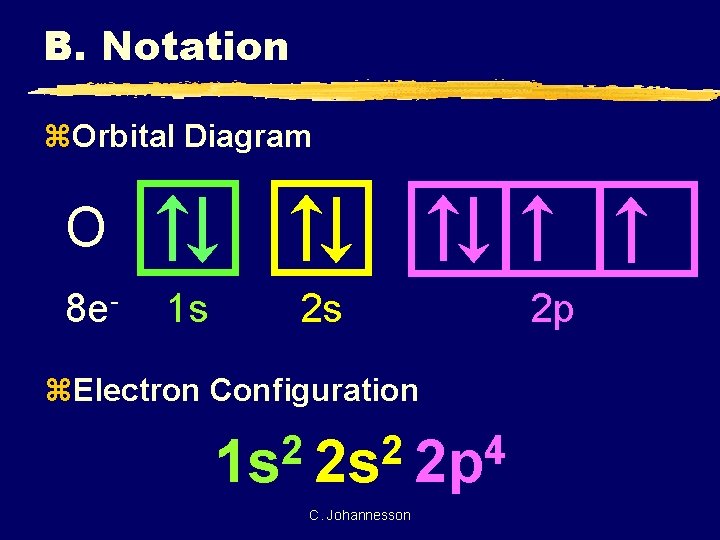
B. Notation z. Orbital Diagram O 8 e- 1 s 2 s z. Electron Configuration 2 2 4 1 s 2 s 2 p C. Johannesson 2 p

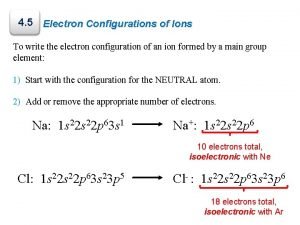
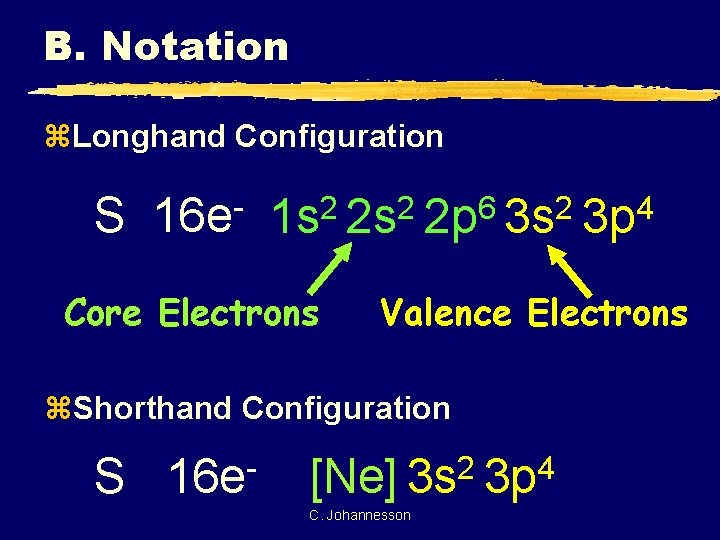
B. Notation z. Longhand Configuration S 16 e 6 2 2 2 1 s 2 s 2 p 3 s Core Electrons Valence Electrons z. Shorthand Configuration S 16 e 4 3 p 2 4 [Ne] 3 s 3 p C. Johannesson

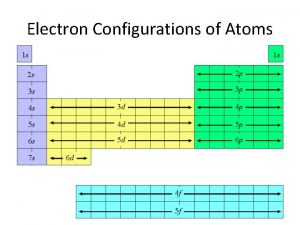
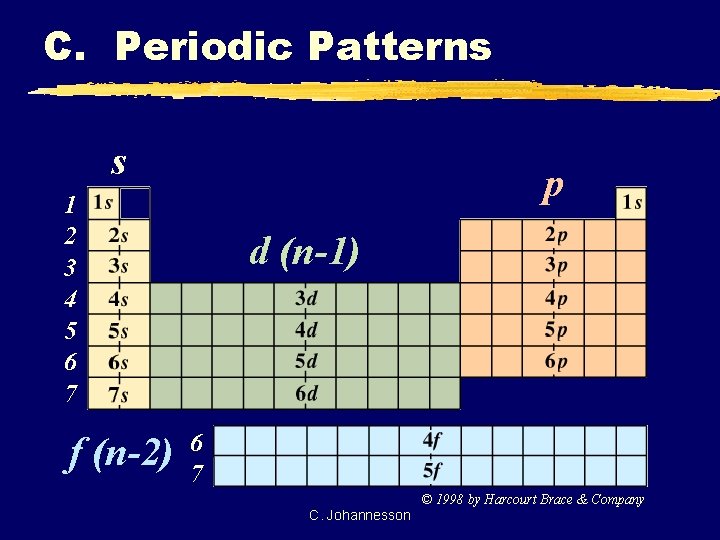
C. Periodic Patterns s p 1 2 3 4 5 6 7 f (n-2) d (n-1) 6 7 © 1998 by Harcourt Brace & Company C. Johannesson

C. Periodic Patterns z. Period # yenergy level (subtract for d & f) z. A/B Group # ytotal # of valence ez. Column within sublevel block y# of e- in sublevel C. Johannesson

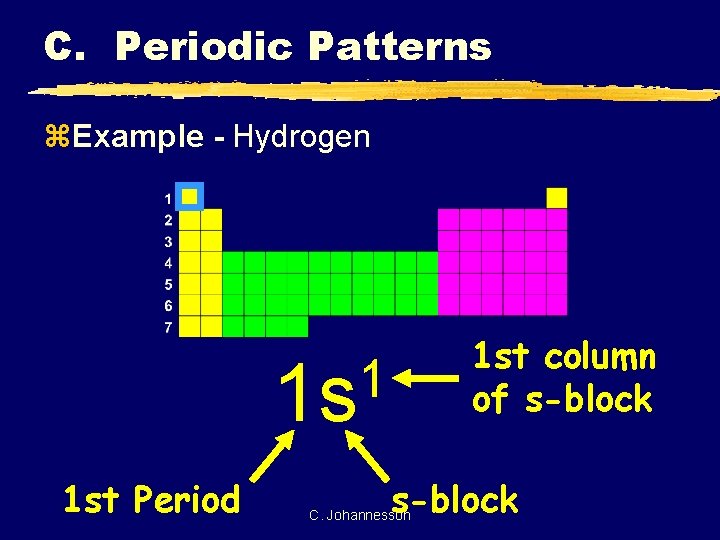
C. Periodic Patterns z. Example - Hydrogen 1 st column of s-block 1 1 s 1 st Period s-block C. Johannesson

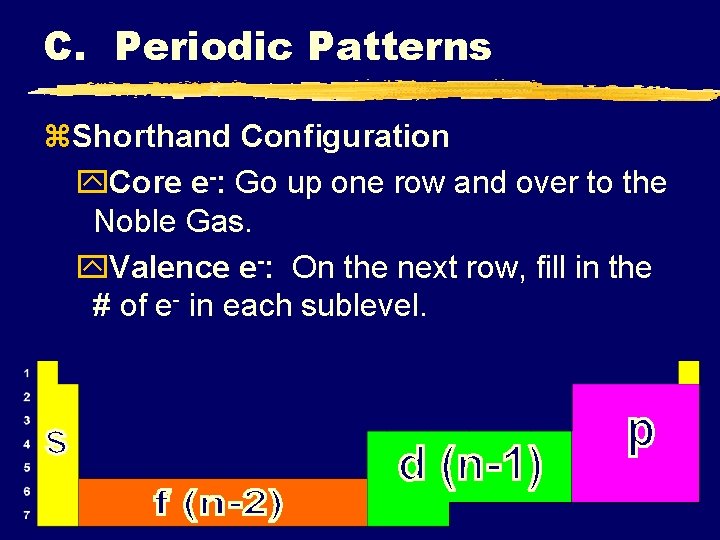
C. Periodic Patterns z. Shorthand Configuration y. Core e-: Go up one row and over to the Noble Gas. y. Valence e-: On the next row, fill in the # of e- in each sublevel. C. Johannesson
![C Periodic Patterns z Example Germanium Ar 2 4 s 10 3 d C. Periodic Patterns z. Example - Germanium [Ar] 2 4 s 10 3 d](https://slidetodoc.com/presentation_image_h/df8713bd517ba0a975c9792279696946/image-14.jpg)
C. Periodic Patterns z. Example - Germanium [Ar] 2 4 s 10 3 d C. Johannesson 2 4 p

D. Stability z. Full energy level z. Full sublevel (s, p, d, f) z. Half-full sublevel C. Johannesson
![D Stability z Electron Configuration Exceptions y Copper EXPECT Ar 4 s 2 3 D. Stability z. Electron Configuration Exceptions y. Copper EXPECT: [Ar] 4 s 2 3](https://slidetodoc.com/presentation_image_h/df8713bd517ba0a975c9792279696946/image-16.jpg)
D. Stability z. Electron Configuration Exceptions y. Copper EXPECT: [Ar] 4 s 2 3 d 9 ACTUALLY: [Ar] 4 s 1 3 d 10 y. Copper gains stability with a full d-sublevel. C. Johannesson
![D Stability z Electron Configuration Exceptions y Chromium EXPECT Ar 4 s 2 3 D. Stability z. Electron Configuration Exceptions y. Chromium EXPECT: [Ar] 4 s 2 3](https://slidetodoc.com/presentation_image_h/df8713bd517ba0a975c9792279696946/image-17.jpg)
D. Stability z. Electron Configuration Exceptions y. Chromium EXPECT: [Ar] 4 s 2 3 d 4 ACTUALLY: [Ar] 4 s 1 3 d 5 y. Chromium gains stability with a half-full d-sublevel. C. Johannesson

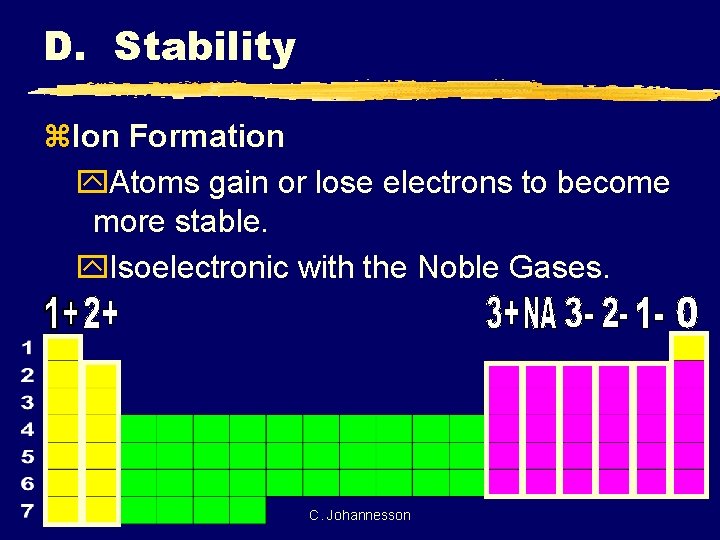
D. Stability z. Ion Formation y. Atoms gain or lose electrons to become more stable. y. Isoelectronic with the Noble Gases. C. Johannesson

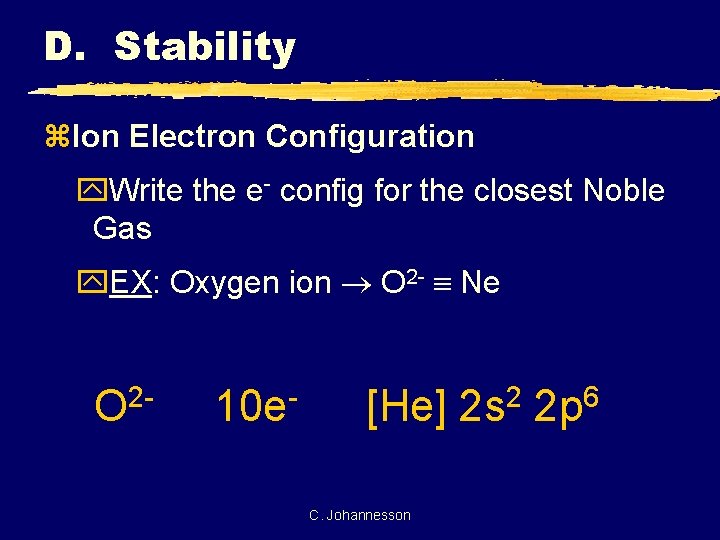
D. Stability z. Ion Electron Configuration y. Write the e- config for the closest Noble Gas y. EX: Oxygen ion O 2 - Ne 2 O 10 e [He] C. Johannesson 2 2 s 6 2 p
 1s 22 s22 p63 s23 p64 s2
1s 22 s22 p63 s23 p64 s2 Electronic configuration of mo (z=42)
Electronic configuration of mo (z=42) Copper subshell configuration
Copper subshell configuration Stable electron configurations are likely to contain
Stable electron configurations are likely to contain Stable electron configurations are likely to contain
Stable electron configurations are likely to contain Stable electron configurations are likely to contain
Stable electron configurations are likely to contain Electron configuration of ions
Electron configuration of ions Stable electron configurations
Stable electron configurations Pictures
Pictures Ap chemistry chapter 7
Ap chemistry chapter 7 Ap chemistry chapter 7 atomic structure and periodicity
Ap chemistry chapter 7 atomic structure and periodicity Chapter 7 atomic structure and periodicity
Chapter 7 atomic structure and periodicity What is periodicity?
What is periodicity? First dental home visit documentation form
First dental home visit documentation form Stenophagic adalah
Stenophagic adalah Chemsheets periodicity
Chemsheets periodicity Bright futures screening guidelines
Bright futures screening guidelines Repeating trends in element properties
Repeating trends in element properties Lymphatic filariasis
Lymphatic filariasis Figure 10
Figure 10