ELECTRON CONFIGURATIONS and PERIODIC PROPERTIES MAIN GROUP ELEMENTS






- Slides: 16

ELECTRON CONFIGURATIONS and PERIODIC PROPERTIES

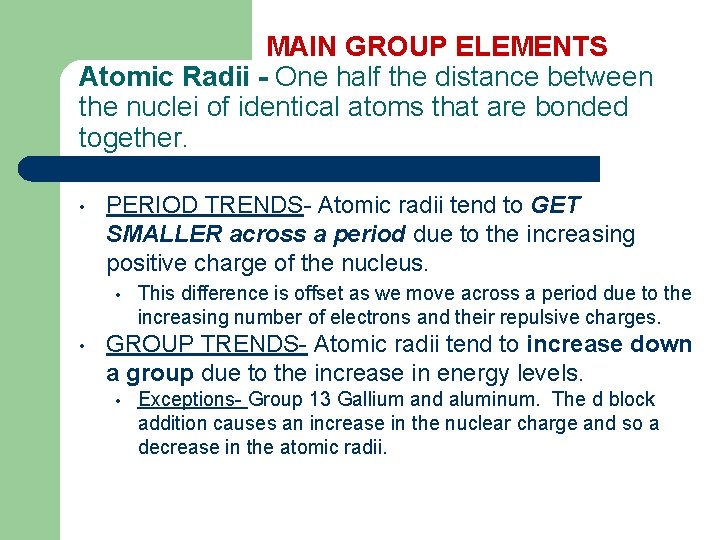
MAIN GROUP ELEMENTS Atomic Radii - One half the distance between the nuclei of identical atoms that are bonded together. • PERIOD TRENDS- Atomic radii tend to GET SMALLER across a period due to the increasing positive charge of the nucleus. • • This difference is offset as we move across a period due to the increasing number of electrons and their repulsive charges. GROUP TRENDS- Atomic radii tend to increase down a group due to the increase in energy levels. • Exceptions- Group 13 Gallium and aluminum. The d block addition causes an increase in the nuclear charge and so a decrease in the atomic radii.

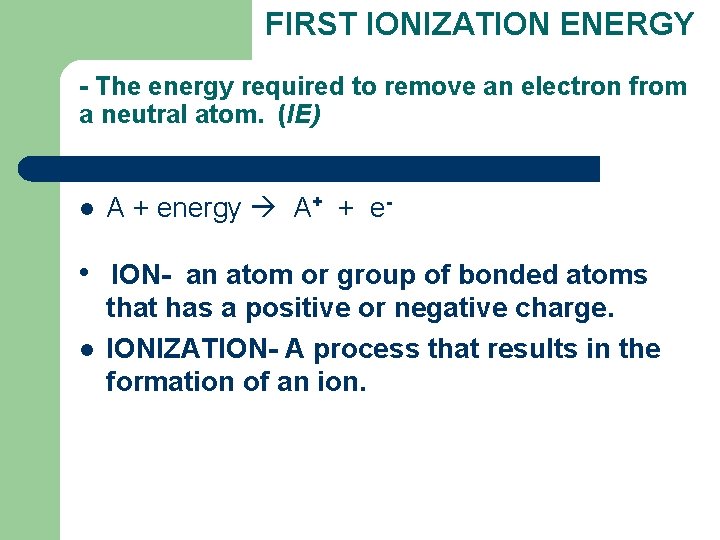
FIRST IONIZATION ENERGY - The energy required to remove an electron from a neutral atom. (IE) l A + energy A+ + e- l ION- an atom or group of bonded atoms that has a positive or negative charge. IONIZATION- A process that results in the formation of an ion. l

Ionization energy continued l l PERIOD TRENDS- In general, IE of the maingroup elements tends to INCREASE across a period. Again caused by increasing nuclear charge. Group 1 -lowest, Group 18 -highest. GROUP TRENDS- In general, IE of the maingroup elements tends to DECREASE down a group because the electrons are further from the nucleus.

Second, Third (etc) Ionization Energies (IE 2 , IE 3 , etc) l l l Each successive removal of an electron requires greater energy because the nucleus has a greater pull on the remaining electrons. SO…the IE tends to INCREASE across a period. At noble- gas configuration, the IE takes a huge jump. EX: Be IE 2 and IE 3 differences. 1757 k. J/mole 14849 k. J/mole

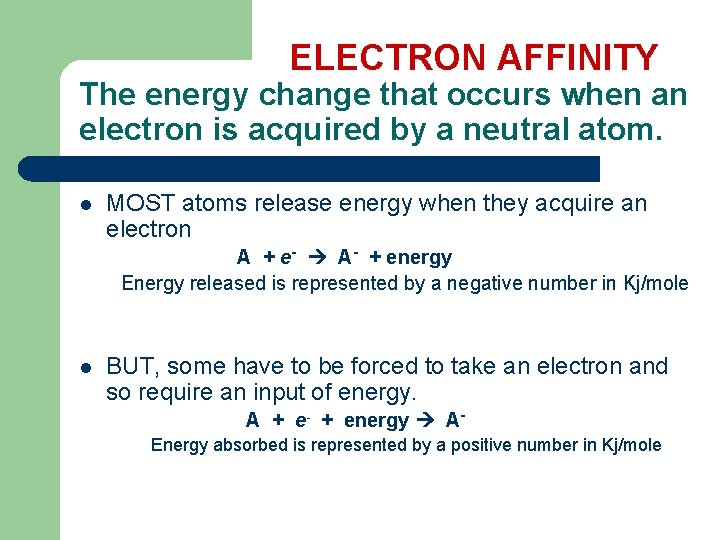
ELECTRON AFFINITY The energy change that occurs when an electron is acquired by a neutral atom. l MOST atoms release energy when they acquire an electron A + e- A- + energy Energy released is represented by a negative number in Kj/mole l BUT, some have to be forced to take an electron and so require an input of energy. A + e- + energy AEnergy absorbed is represented by a positive number in Kj/mole

ELECTRON AFFINITY continued l PERIOD TRENDS- Affinity becomes MORE NEGATIVE across a period. – l Exceptions- Group 14 and 15 because adding an electron to group 14 elements gives a half-full p orbital, while adding an electron to group 15 requires the pairing of electrons in the p sublevel. GROUP TRENDS- Generally becoming LESS NEGATIVE down a group due to increase in atomic radius, so less pull from the nucleus, even though the pull is a little stronger – Exceptions- heavy transition metals.

ELECTRON AFFINITY continued l The addition of a second electron to an already negative ion must always be forced, so second electron affinities are all positive values.

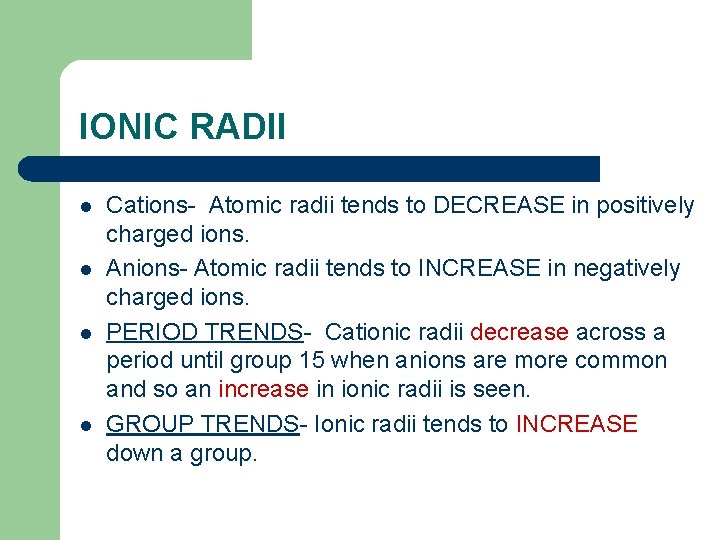
IONIC RADII l l Cations- Atomic radii tends to DECREASE in positively charged ions. Anions- Atomic radii tends to INCREASE in negatively charged ions. PERIOD TRENDS- Cationic radii decrease across a period until group 15 when anions are more common and so an increase in ionic radii is seen. GROUP TRENDS- Ionic radii tends to INCREASE down a group.

VALENCE ELECTRONS l The electrons available to be lost, gained or shared in the formation of chemical compounds.

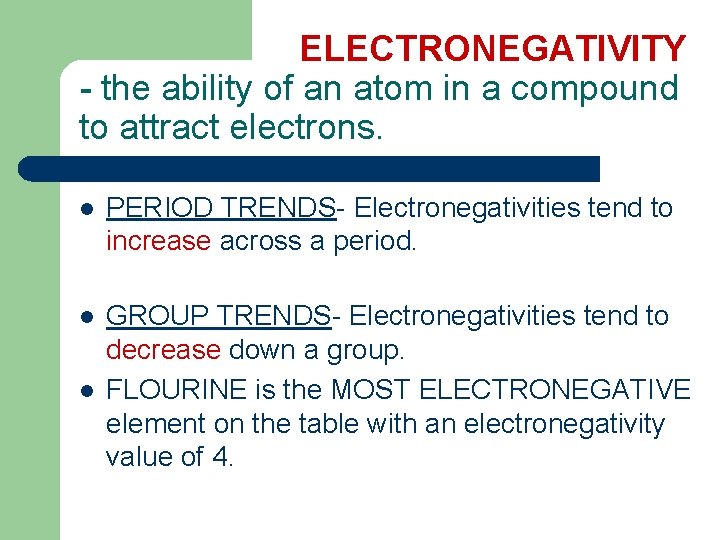
ELECTRONEGATIVITY - the ability of an atom in a compound to attract electrons. l PERIOD TRENDS- Electronegativities tend to increase across a period. l GROUP TRENDS- Electronegativities tend to decrease down a group. FLOURINE is the MOST ELECTRONEGATIVE element on the table with an electronegativity value of 4. l

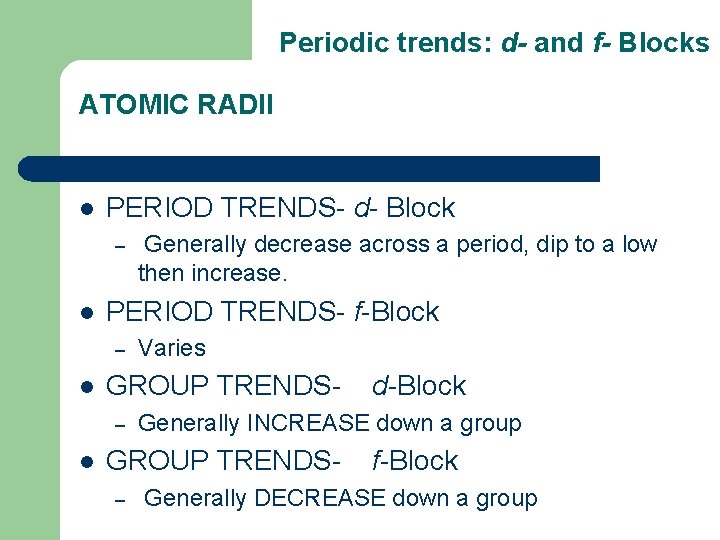
Periodic trends: d- and f- Blocks ATOMIC RADII l PERIOD TRENDS- d- Block – l PERIOD TRENDS- f-Block – l Varies GROUP TRENDS– l Generally decrease across a period, dip to a low then increase. Generally INCREASE down a group GROUP TRENDS– d-Block f-Block Generally DECREASE down a group

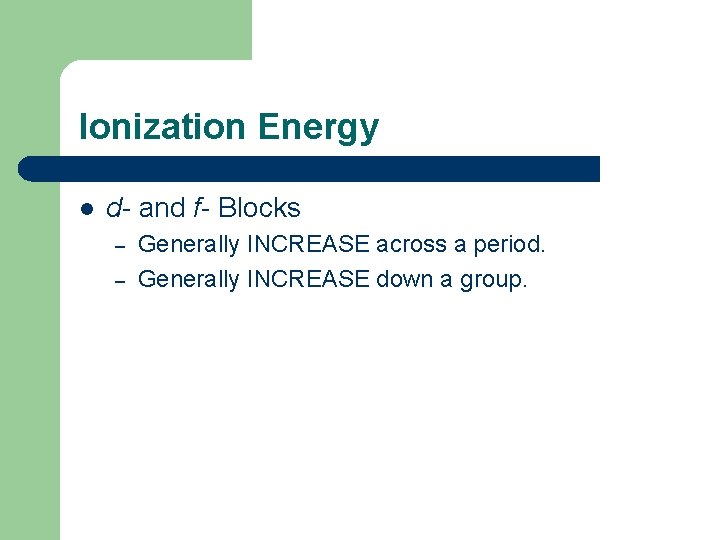
Ionization Energy l d- and f- Blocks – – Generally INCREASE across a period. Generally INCREASE down a group.

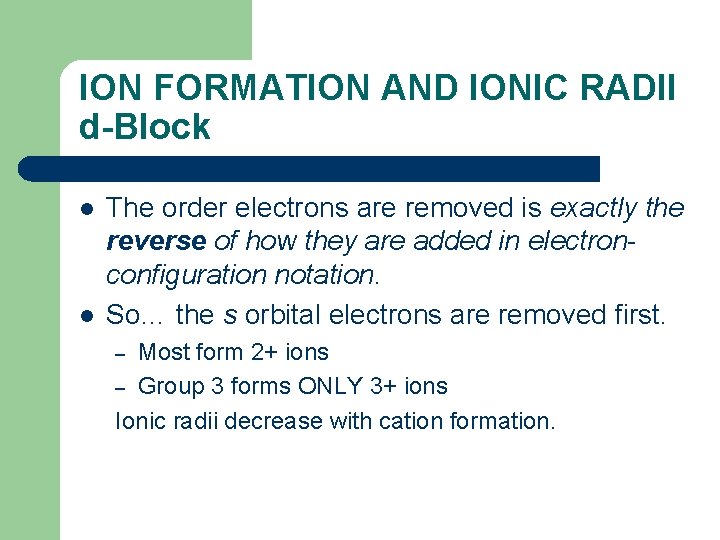
ION FORMATION AND IONIC RADII d-Block l l The order electrons are removed is exactly the reverse of how they are added in electronconfiguration notation. So… the s orbital electrons are removed first. Most form 2+ ions – Group 3 forms ONLY 3+ ions Ionic radii decrease with cation formation. –

ELECTRONEGATIVITIES l Only Group 1 and 2 have lower electronegativities than d- or f- Block elements l D-Block has higher electronegativities than f. Block generally
