Electron Configurations and Orbital Notation Diagrams Helps predict
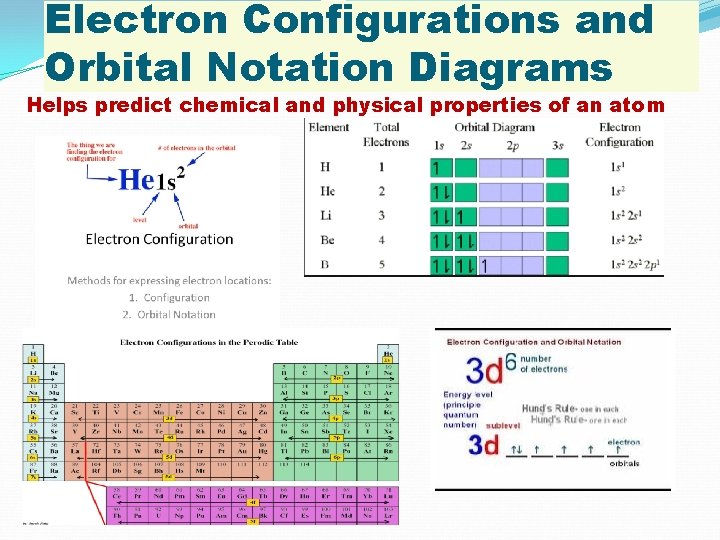
Electron Configurations and Orbital Notation Diagrams Helps predict chemical and physical properties of an atom
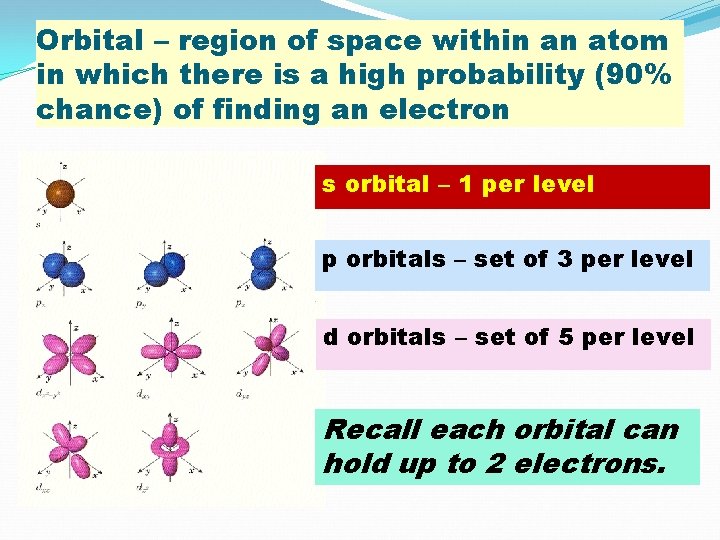
Orbital – region of space within an atom in which there is a high probability (90% chance) of finding an electron s orbital – 1 per level p orbitals – set of 3 per level d orbitals – set of 5 per level Recall each orbital can hold up to 2 electrons.
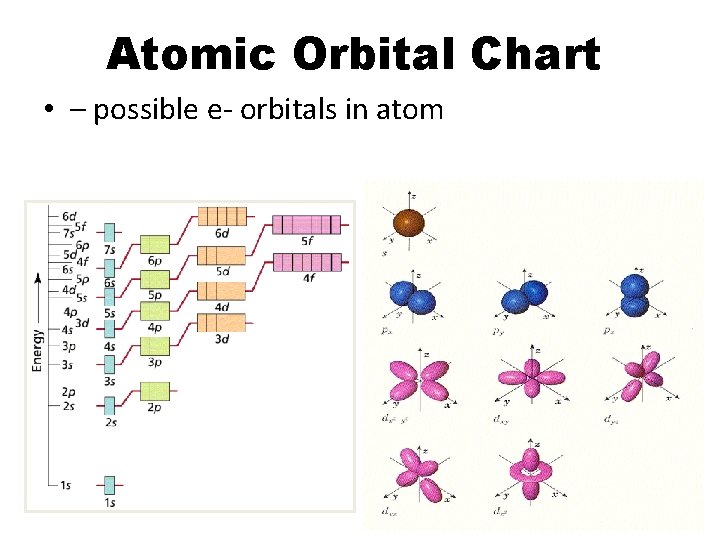
Atomic Orbital Chart • – possible e- orbitals in atom
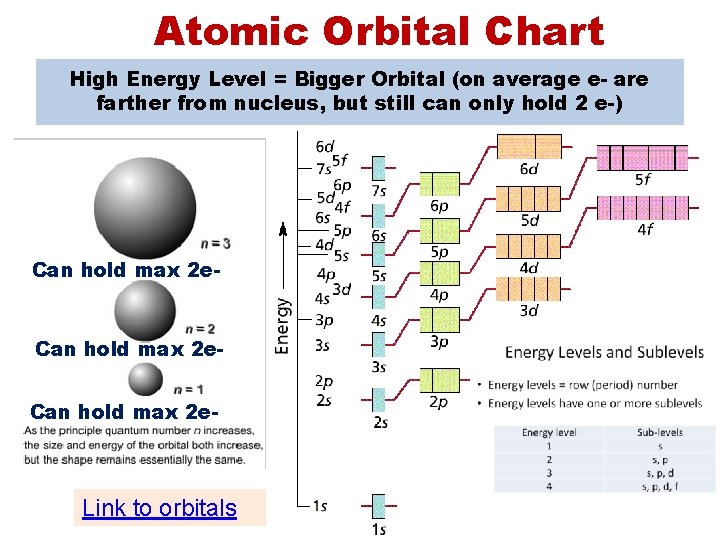
Atomic Orbital Chart High Energy Level = Bigger Orbital (on average e- are farther from nucleus, but still can only hold 2 e-) Can hold max 2 e- Link to orbitals
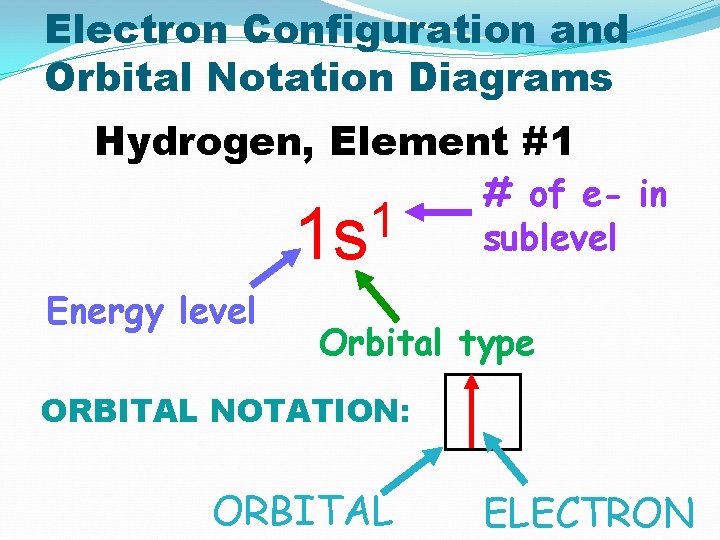
Electron Configuration and Orbital Notation Diagrams Hydrogen, Element #1 1 1 s Energy level # of e- in sublevel Orbital type ORBITAL NOTATION: ORBITAL ELECTRON
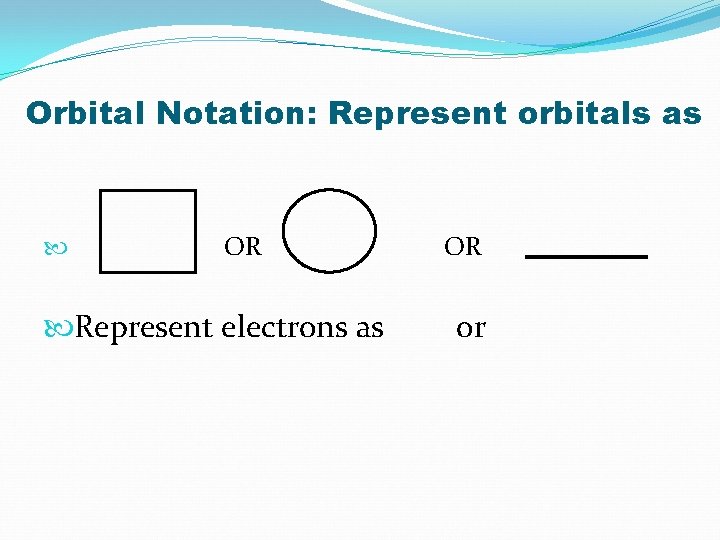
Orbital Notation: Represent orbitals as OR Represent electrons as OR or
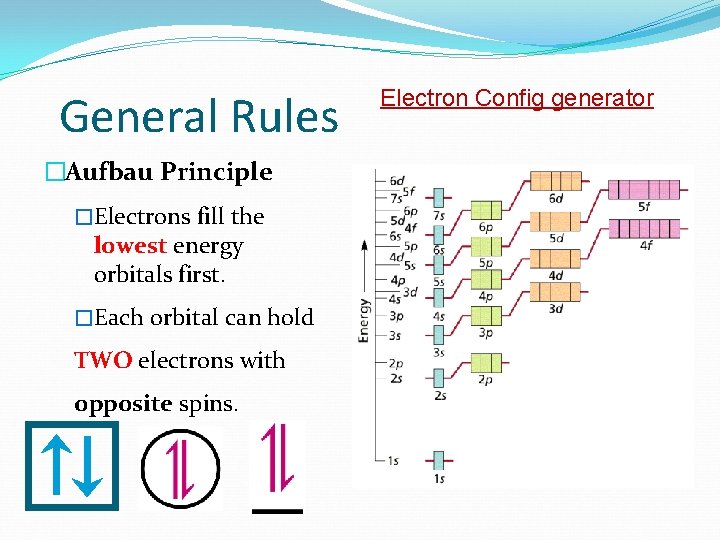
General Rules �Aufbau Principle �Electrons fill the lowest energy orbitals first. �Each orbital can hold TWO electrons with opposite spins. Electron Config generator

General Rules Hund’s Rule Within a sublevel containing more than orbital (p, d or f), place one e- per orbital before pairing them. “Empty Bus Seat Rule” WRONG RIGHT
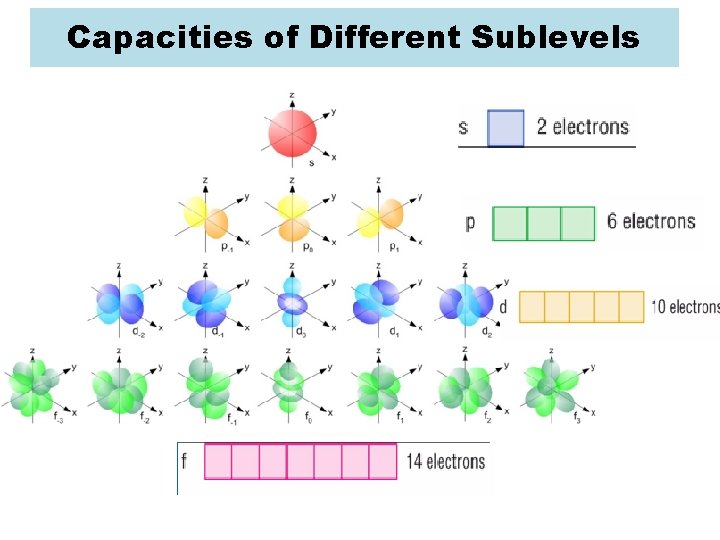
Capacities of Different Sublevels
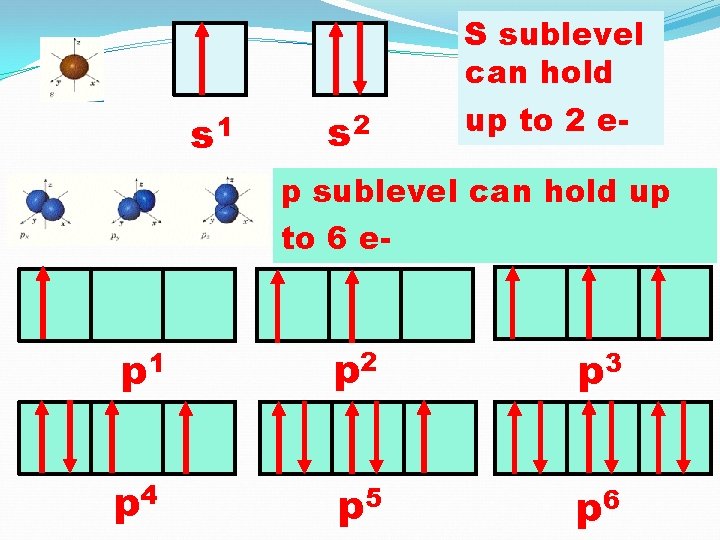
s 1 s 2 S sublevel can hold up to 2 e- p sublevel can hold up to 6 e- p 1 p 2 p 3 p 4 p 5 p 6
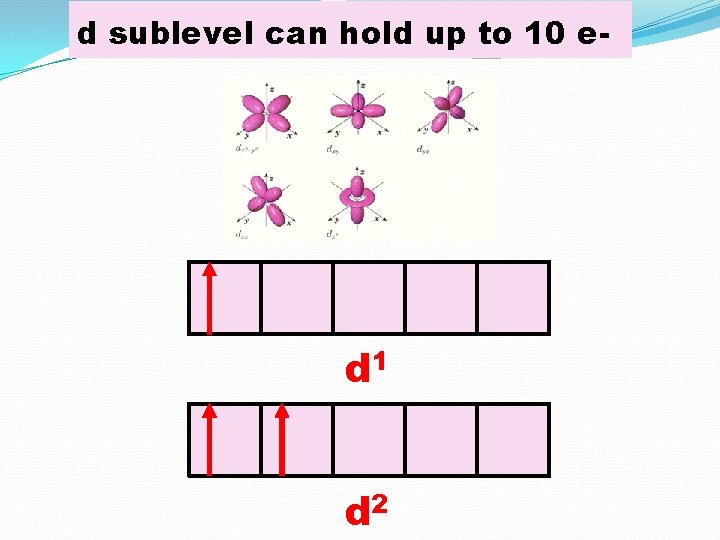
d sublevel can hold up to 10 e- d 1 d 2
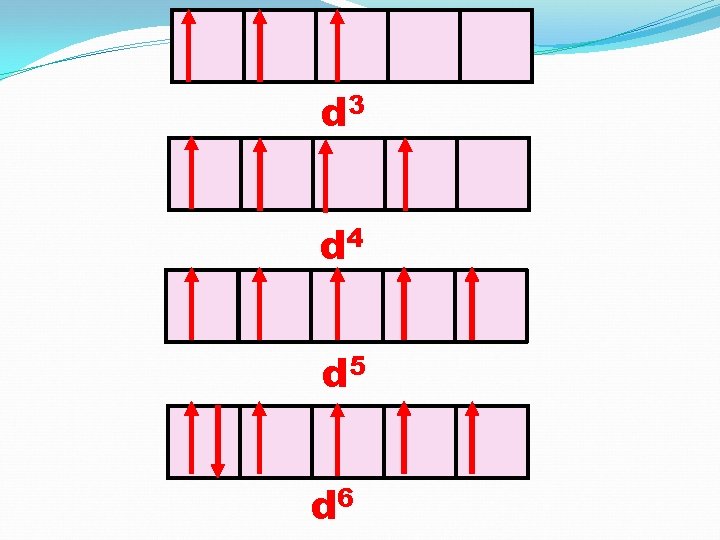
d 3 d 4 d 5 d 6
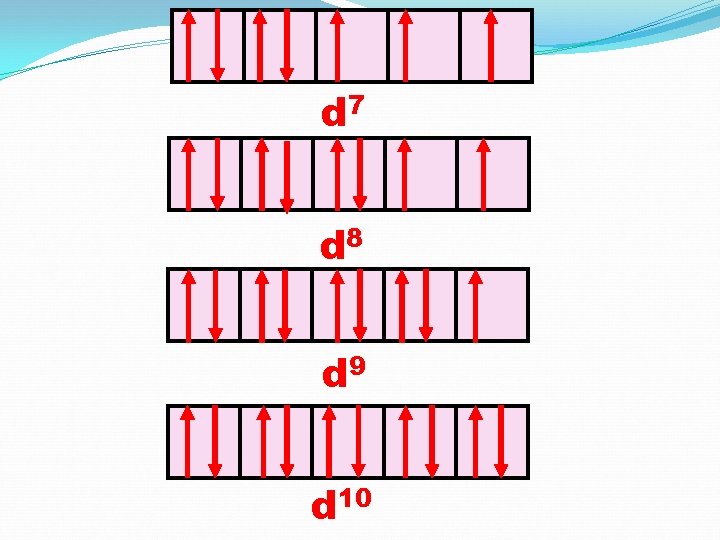
d 7 d 8 d 9 d 10
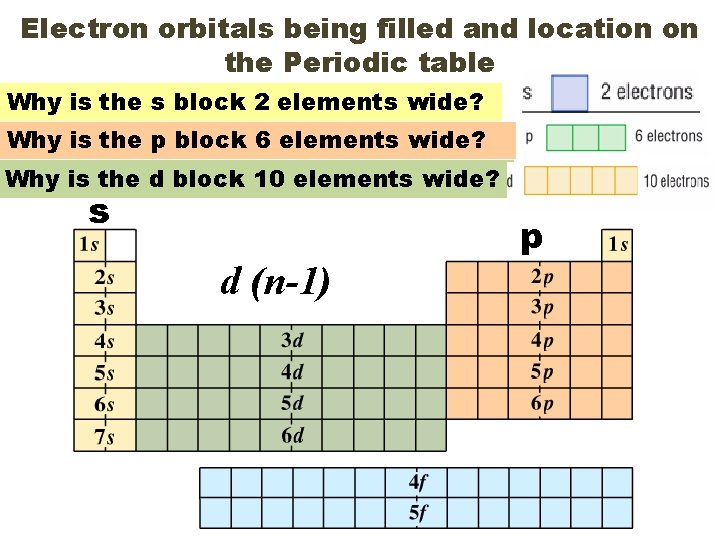
Electron orbitals being filled and location on the Periodic table Why is the s block 2 elements wide? Why is the p block 6 elements wide? Why is the d block 10 elements wide? s d (n-1) p
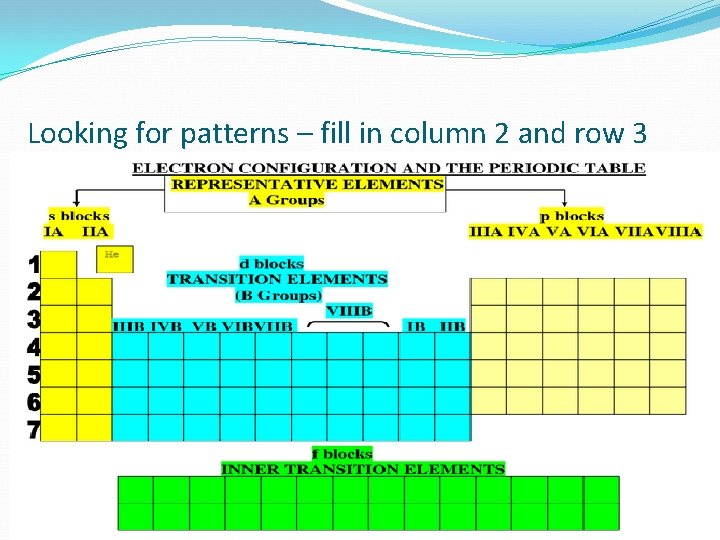
Looking for patterns – fill in column 2 and row 3
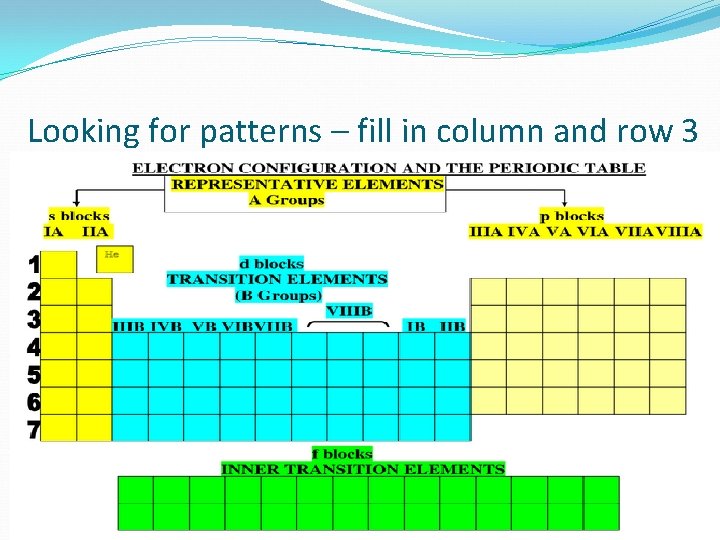
Looking for patterns – fill in column and row 3
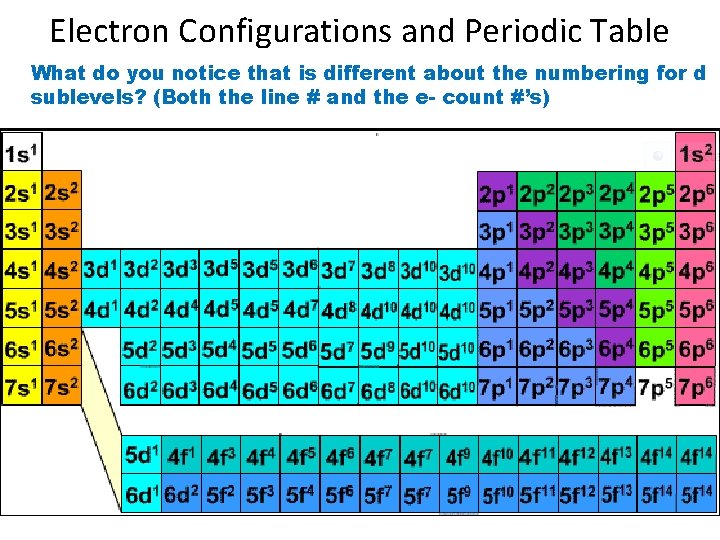
Electron Configurations and Periodic Table What do you notice that is different about the numbering for d sublevels? (Both the line # and the e- count #’s)
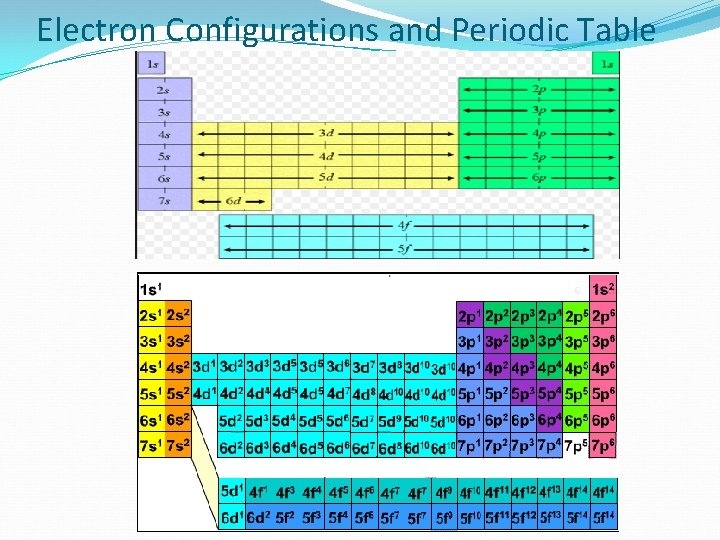
Electron Configurations and Periodic Table
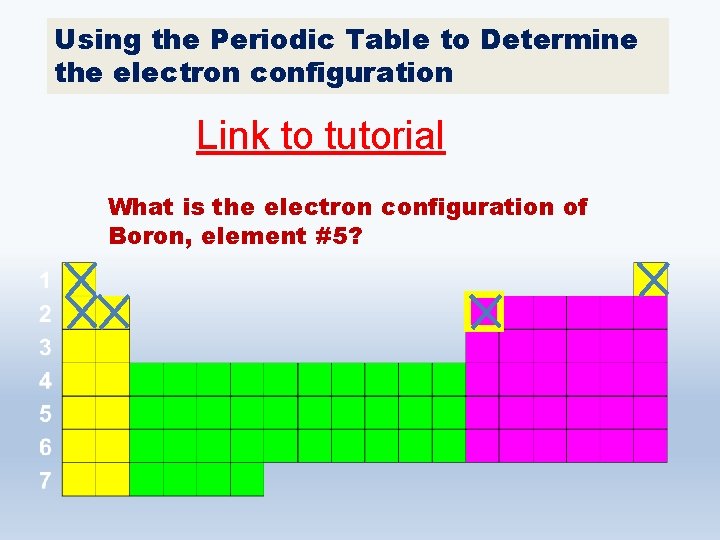
Using the Periodic Table to Determine the electron configuration Link to tutorial What is the electron configuration of Boron, element #5?
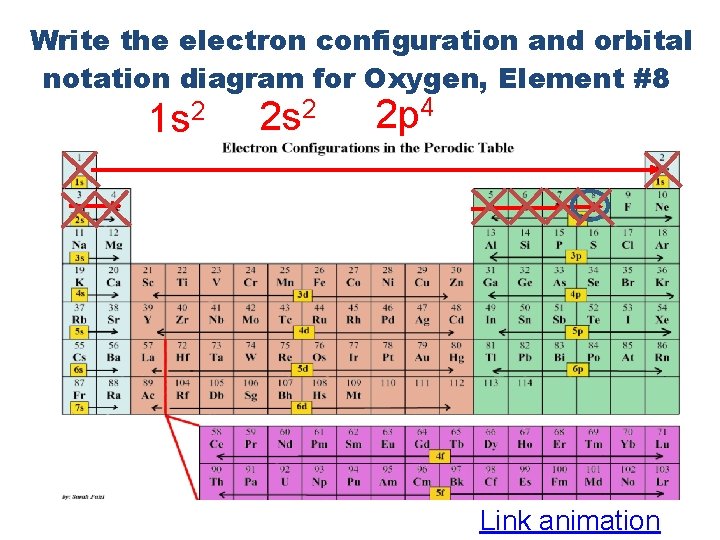
Write the electron configuration and orbital notation diagram for Oxygen, Element #8 1 s 2 2 p 4 Link animation
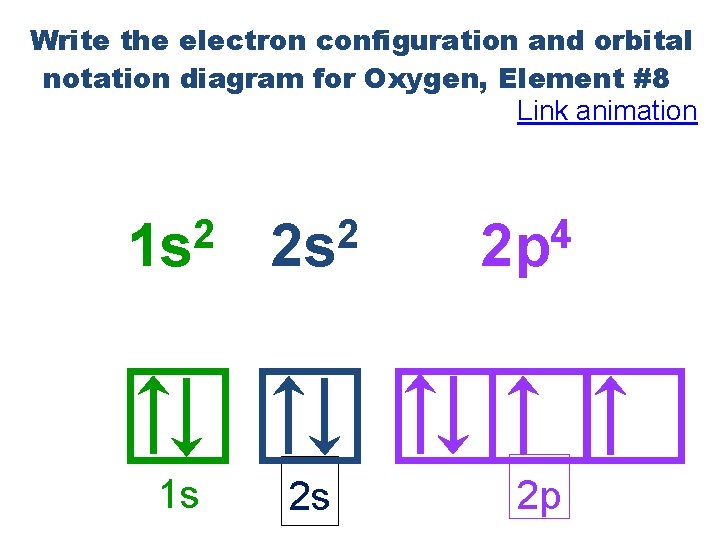
Write the electron configuration and orbital notation diagram for Oxygen, Element #8 Link animation 2 1 s 2 2 s 1 s 2 s 4 2 p 2 p
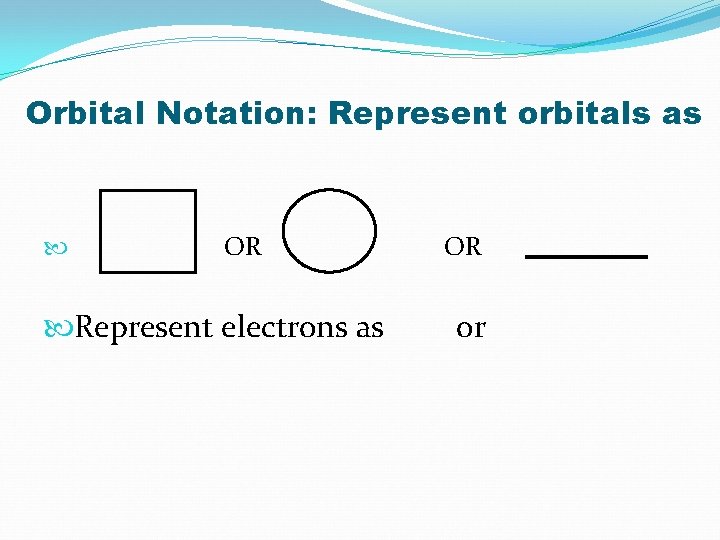
Orbital Notation: Represent orbitals as OR Represent electrons as OR or
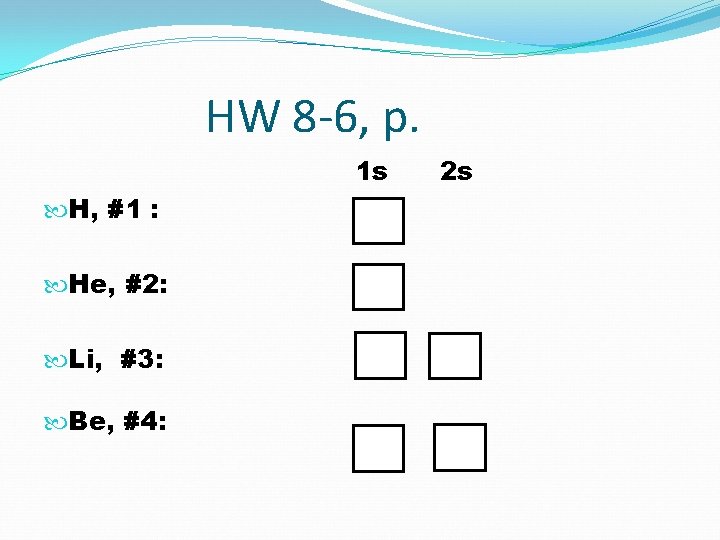
HW 8 -6, p. 1 s H, #1 : He, #2: Li, #3: Be, #4: 2 s
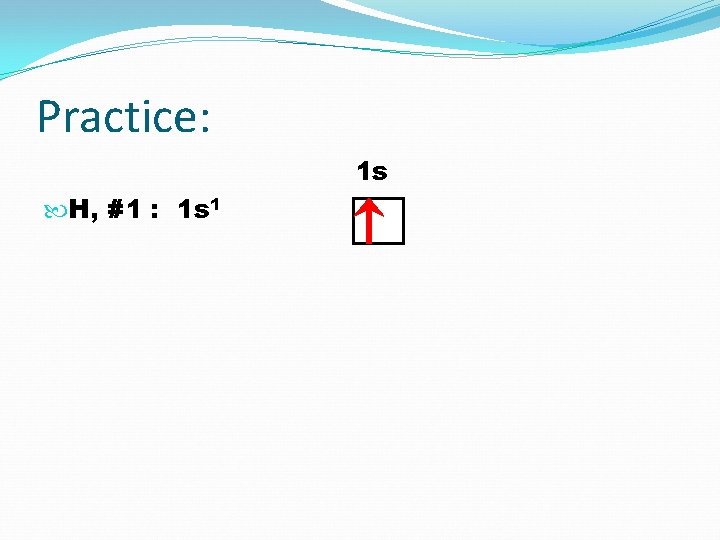
Practice: 1 s H, #1 : 1 s 1
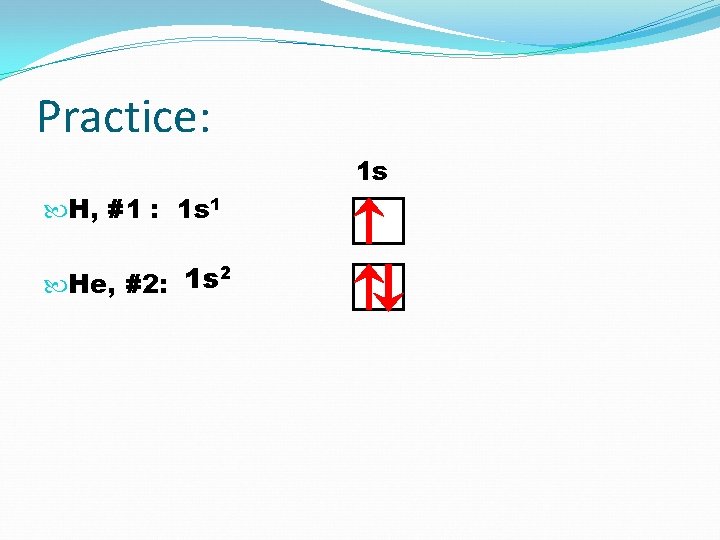
Practice: 1 s H, #1 : 1 s 1 2 1 s He, #2:
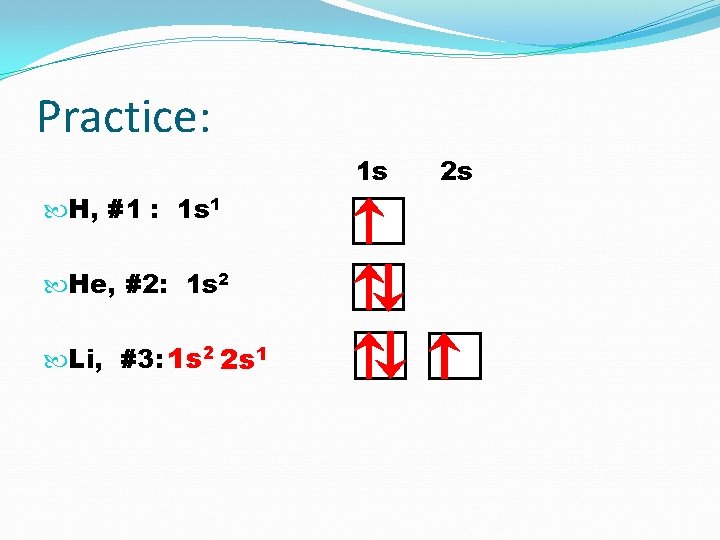
Practice: 1 s H, #1 : 1 s 1 He, #2: 1 s 2 Li, #3: 1 s 2 2 s 1 2 s
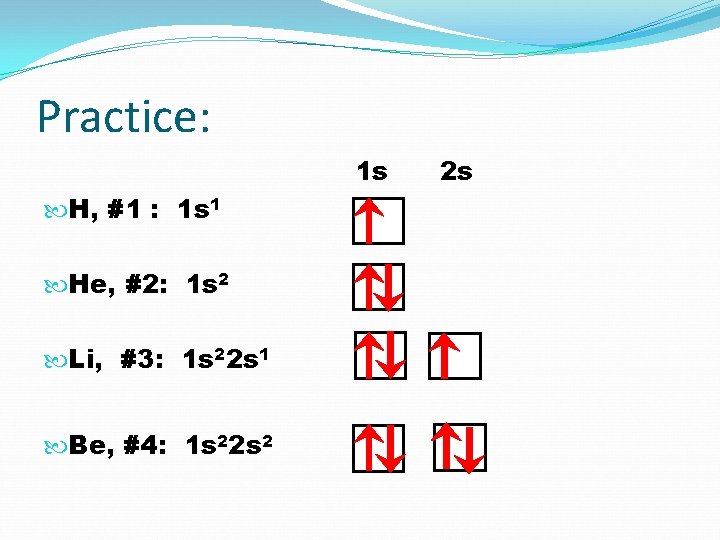
Practice: 1 s H, #1 : 1 s 1 He, #2: 1 s 2 Li, #3: 1 s 22 s 1 Be, #4: 1 s 22 s 2 2 s
Practice: 1 s B, #5 : C, #6: N, #7: O, #8: 2 s 2 p
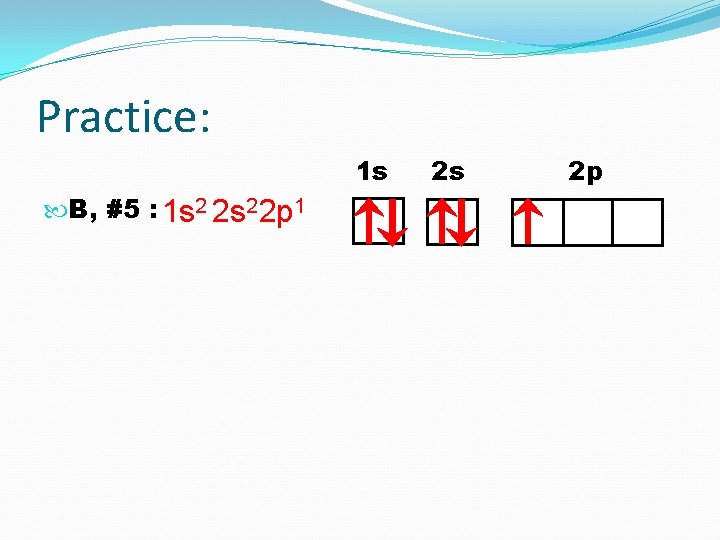
Practice: 1 s B, #5 : 1 s 2 2 p 1 2 s 2 p
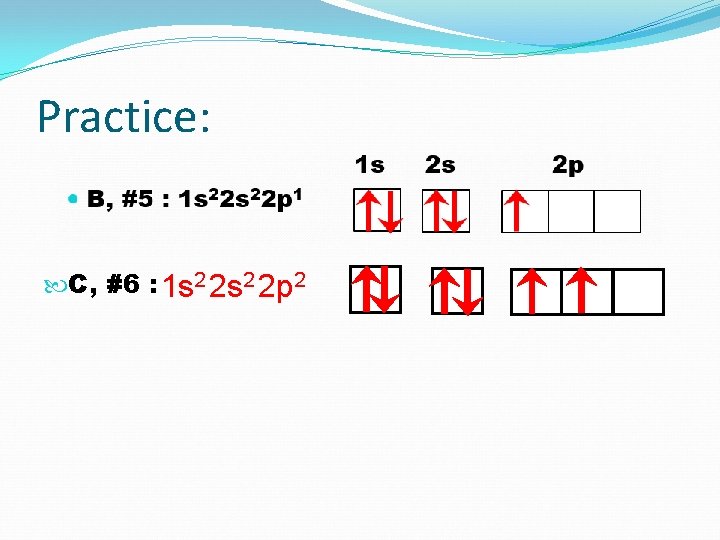
Practice: 1 s C, #6 : 1 s 2 2 p 2 2 s 2 p
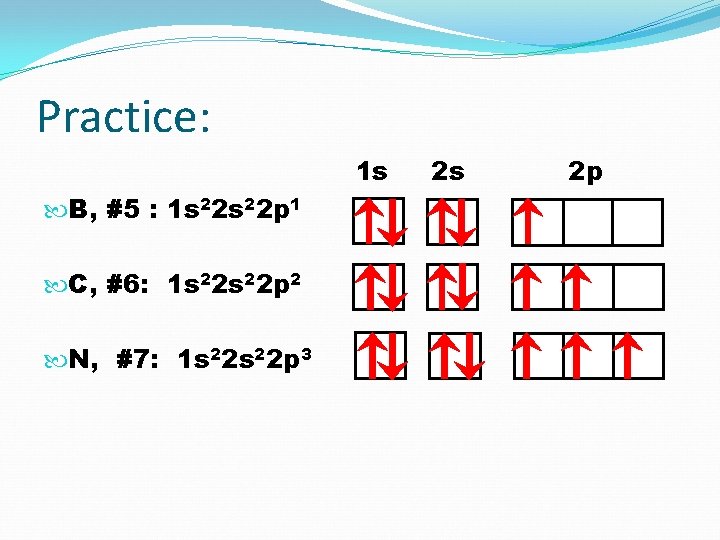
Practice: 1 s B, #5 : 1 s 22 p 1 C, #6: 1 s 22 p 2 N, #7: 1 s 22 p 3 2 s 2 p
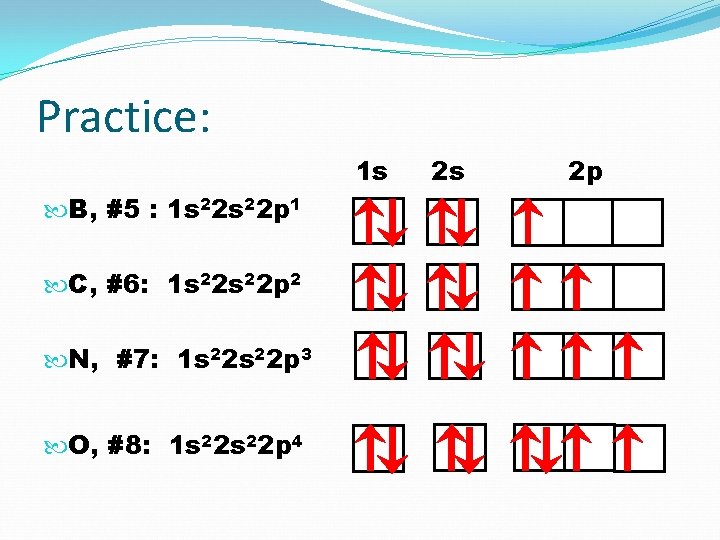
Practice: 1 s B, #5 : 1 s 22 p 1 C, #6: 1 s 22 p 2 N, #7: 1 s 22 p 3 O, #8: 1 s 22 p 4 2 s 2 p
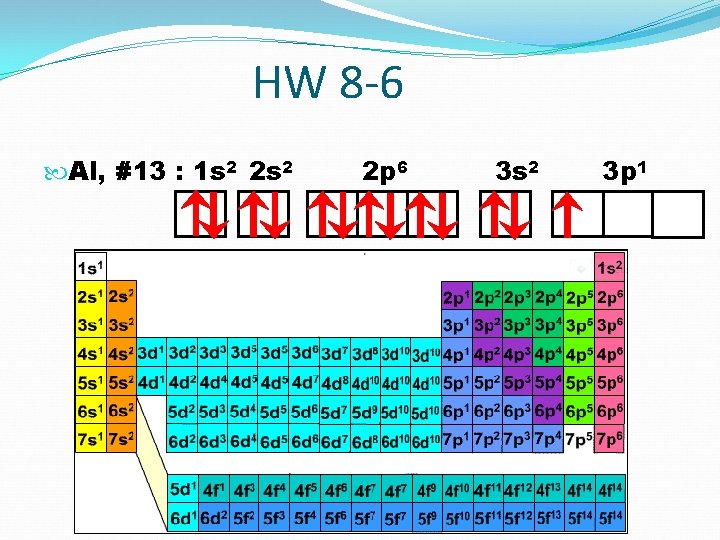
HW 8 -6 Al, #13 : 1 s 2 2 p 6 3 s 2 3 p 1
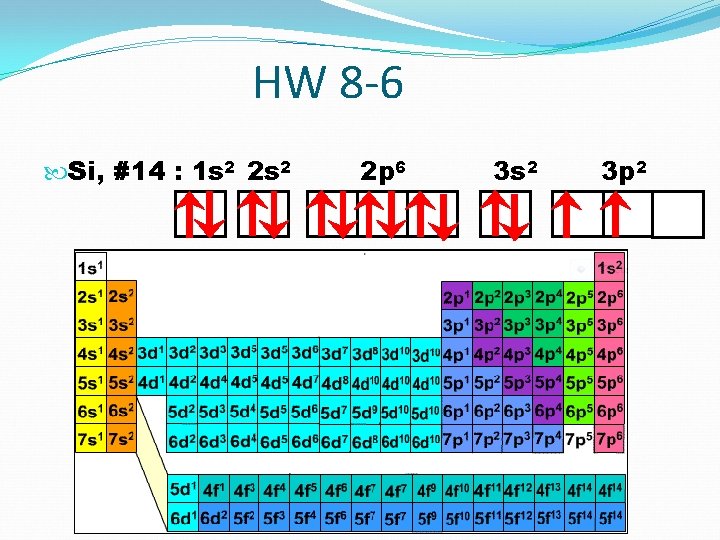
HW 8 -6 Si, #14 : 1 s 2 2 p 6 3 s 2 3 p 2
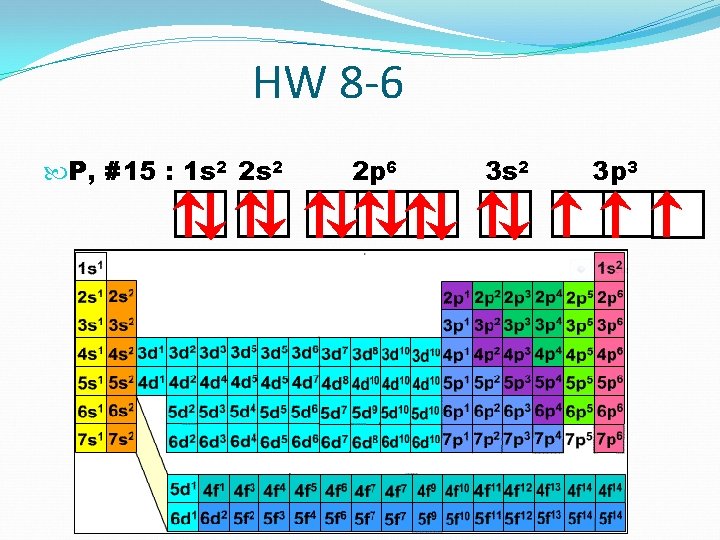
HW 8 -6 P, #15 : 1 s 2 2 p 6 3 s 2 3 p 3
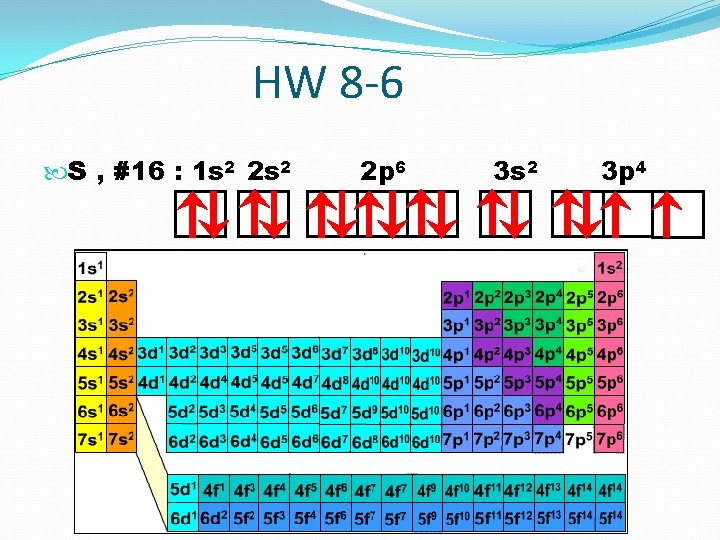
HW 8 -6 S , #16 : 1 s 2 2 p 6 3 s 2 3 p 4
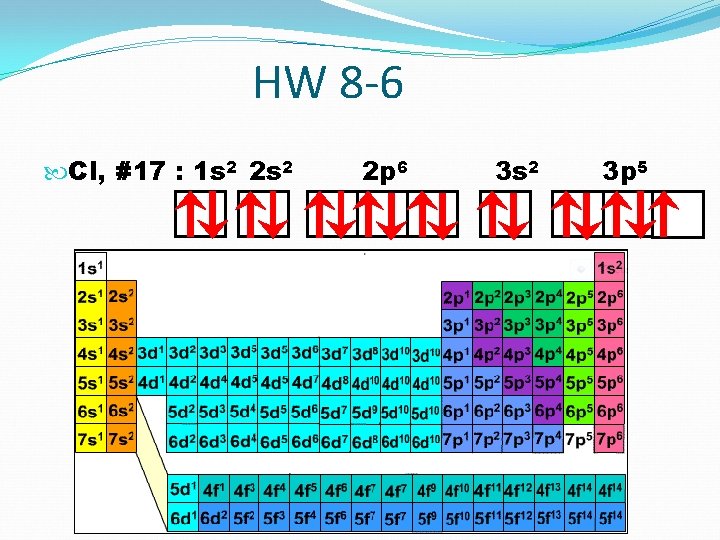
HW 8 -6 Cl, #17 : 1 s 2 2 p 6 3 s 2 3 p 5
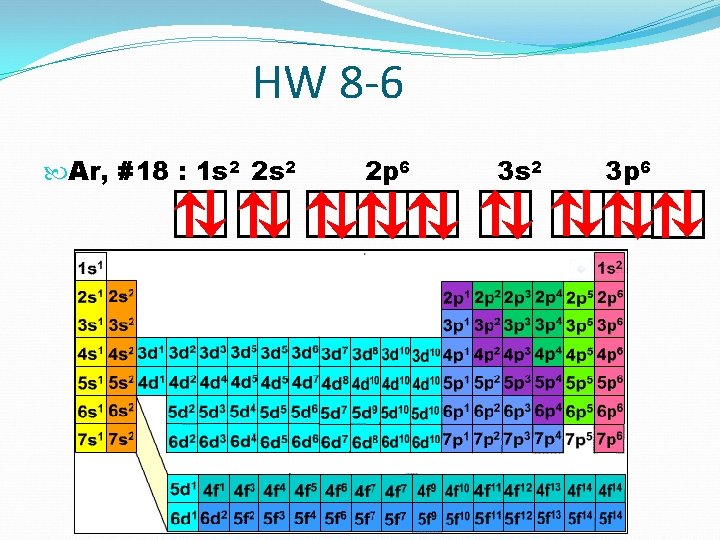
HW 8 -6 Ar, #18 : 1 s 2 2 p 6 3 s 2 3 p 6
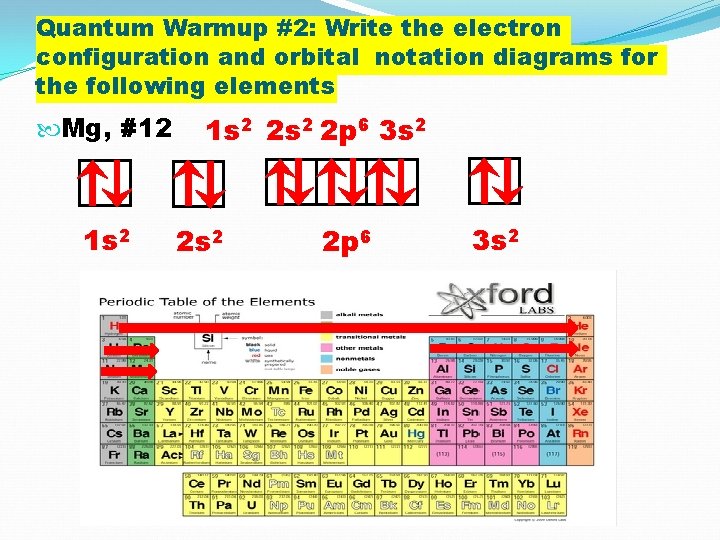
Quantum Warmup #2: Write the electron configuration and orbital notation diagrams for the following elements Mg, #12 1 s 2 2 s 2 2 p 6 3 s 2
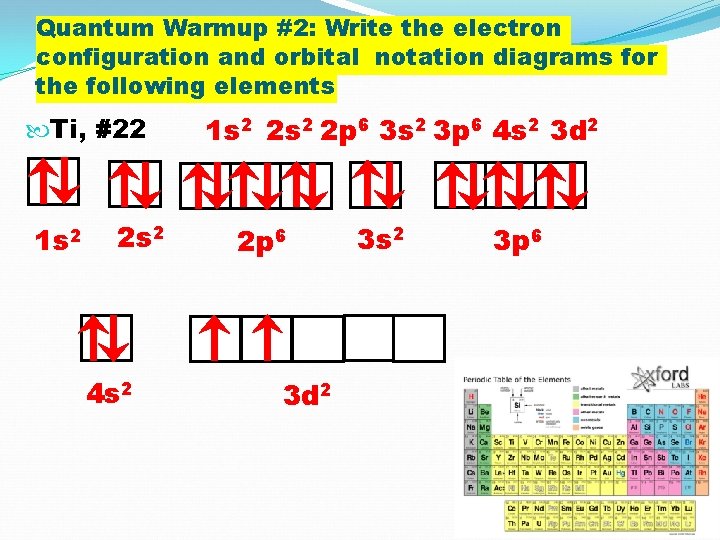
Quantum Warmup #2: Write the electron configuration and orbital notation diagrams for the following elements Ti, #22 1 s 2 2 s 2 4 s 2 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 2 2 p 6 3 d 2 3 s 2 3 p 6
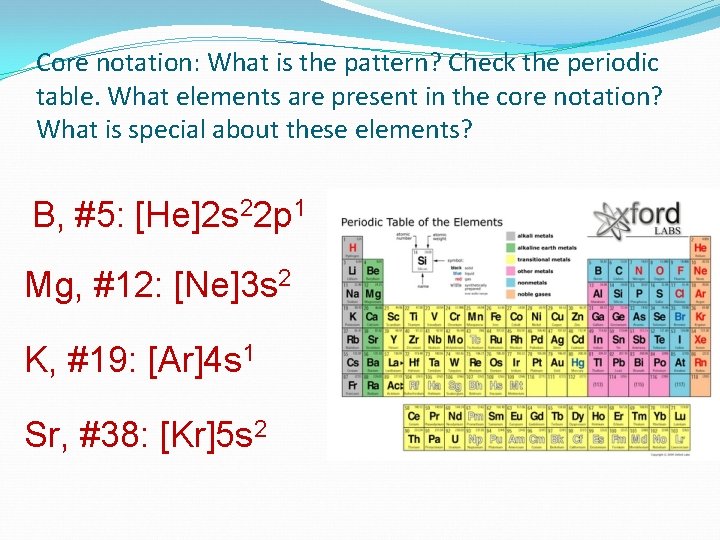
Core notation: What is the pattern? Check the periodic table. What elements are present in the core notation? What is special about these elements? B, #5: [He]2 s 22 p 1 Mg, #12: [Ne]3 s 2 K, #19: [Ar]4 s 1 Sr, #38: [Kr]5 s 2
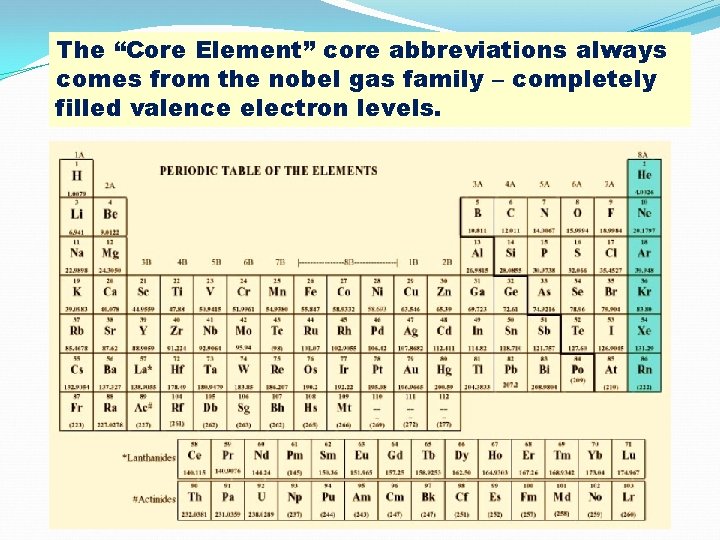
The “Core Element” core abbreviations always comes from the nobel gas family – completely filled valence electron levels.
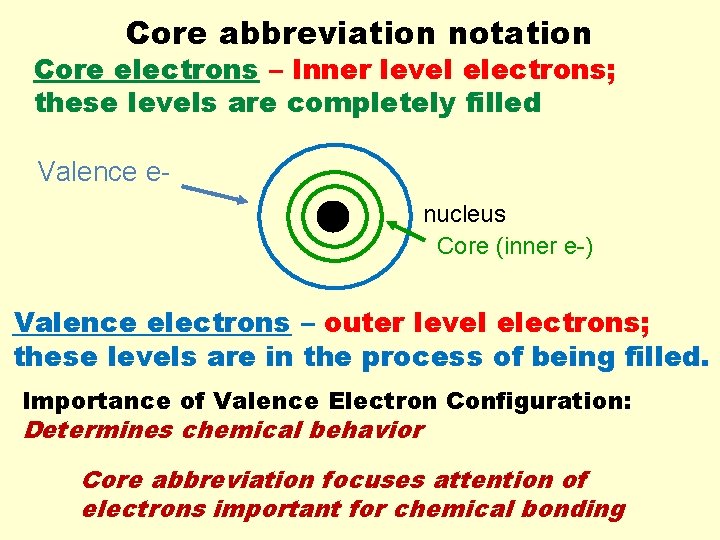
Core abbreviation notation Core electrons – Inner level electrons; these levels are completely filled Valence enucleus Core (inner e-) Valence electrons – outer level electrons; these levels are in the process of being filled. Importance of Valence Electron Configuration: Determines chemical behavior Core abbreviation focuses attention of electrons important for chemical bonding
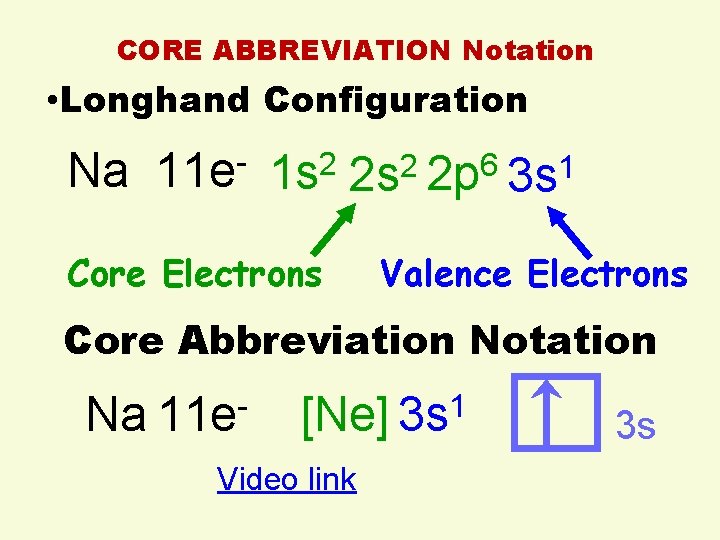
CORE ABBREVIATION Notation • Longhand Configuration Na 11 e- 1 s 2 2 p 6 3 s 1 Core Electrons Valence Electrons Core Abbreviation Notation Na 11 e- [Ne] 3 s 1 Video link 3 s
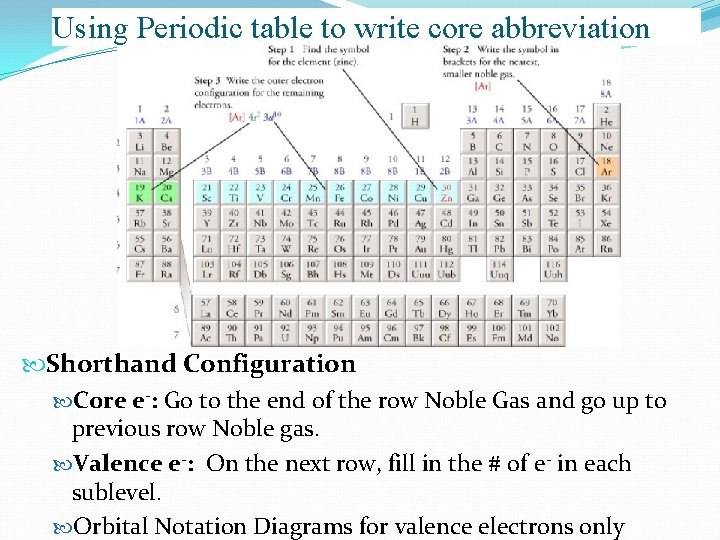
Using Periodic table to write core abbreviation Shorthand Configuration Core e-: Go to the end of the row Noble Gas and go up to previous row Noble gas. Valence e-: On the next row, fill in the # of e- in each sublevel. Orbital Notation Diagrams for valence electrons only
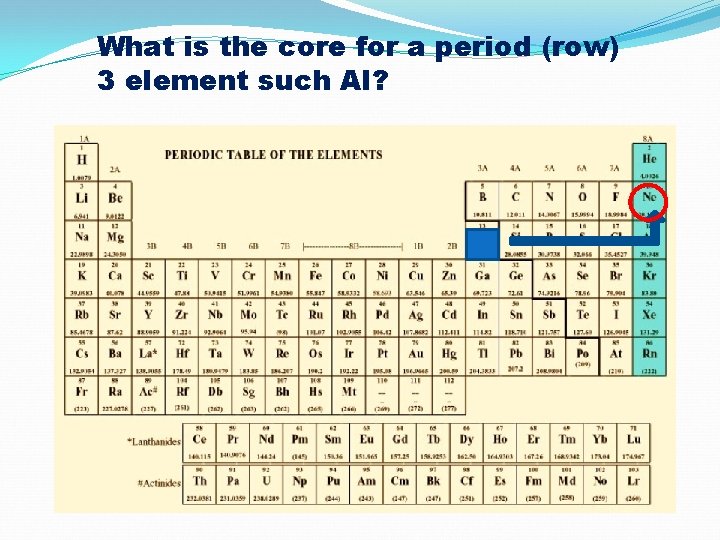
What is the core for a period (row) 3 element such Al?
![What is the core abbreviation notation for Al, #13 [Ne] 3 s 2 3 What is the core abbreviation notation for Al, #13 [Ne] 3 s 2 3](http://slidetodoc.com/presentation_image_h2/932fd9b23a1bd317b82783acf953d7fa/image-47.jpg)
What is the core abbreviation notation for Al, #13 [Ne] 3 s 2 3 p 1
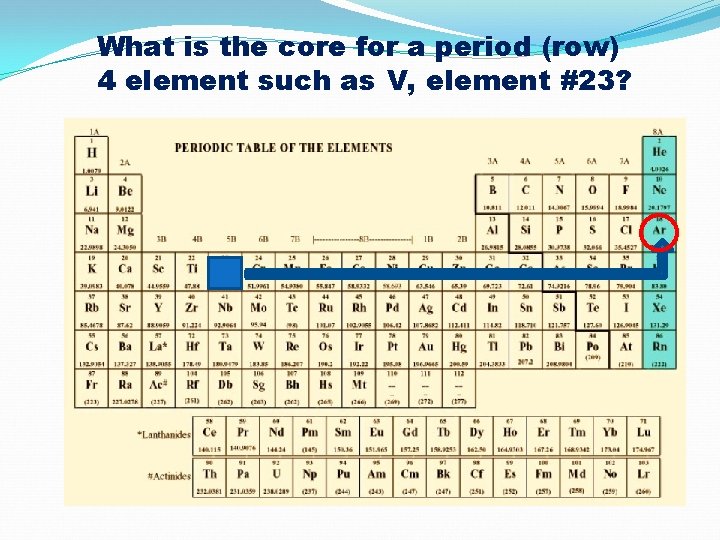
What is the core for a period (row) 4 element such as V, element #23?
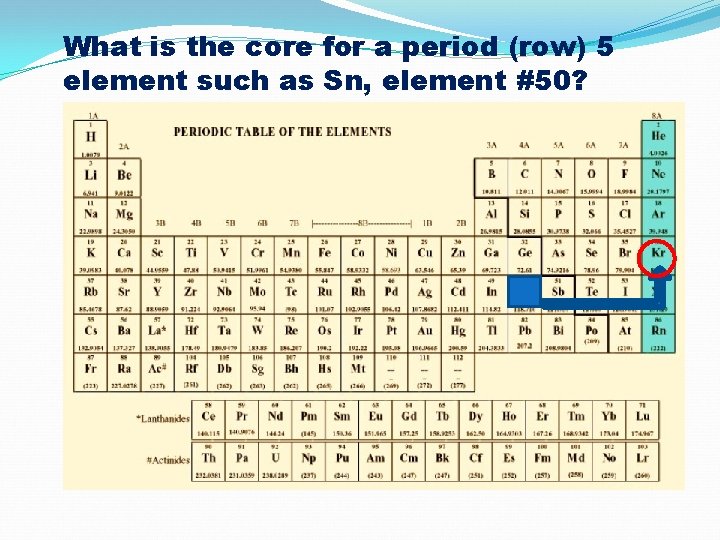
What is the core for a period (row) 5 element such as Sn, element #50?

HW 8 -7 Key Link
- Slides: 50