Electron configurations and Orbital Diagrams What is an
Electron configurations and Orbital Diagrams

What is an electron configuration? It shows how the electrons are placed around an atom. What are they used for? To show an atom will bond and/or to explain the charge an atom has when it becomes an ion.

Number of electrons in that sublevel Energy level 1 2 3 3 s 2 Sublevel Letter of # of orbital s 1 p 3 d 5 f 7 Max # e 2 6 10 14

Order of filling 1 s 2 s 3 s 4 s 5 s 6 s 7 s Notice the number of columns in each section is equal to max number of e- that each sublevel can hold 2 p 3 p 3 d 4 d 4 f 5 f s 4 p 5 p 5 d 6 p 6 d 7 p p d f
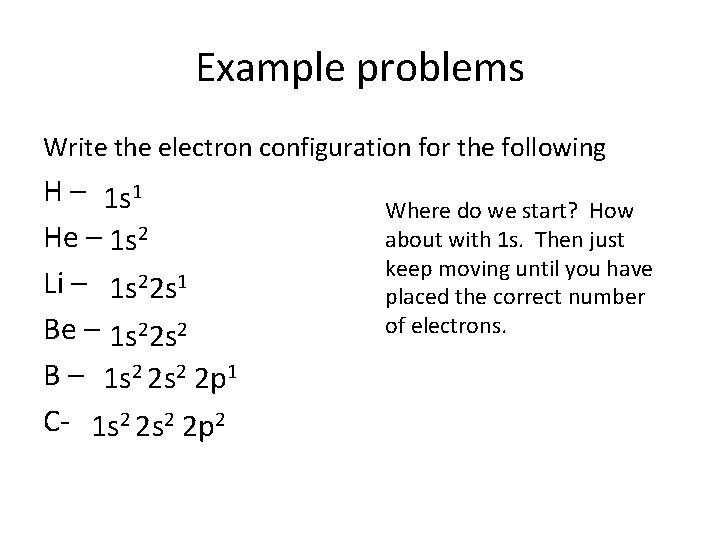
Example problems Write the electron configuration for the following H – 1 s 1 He – 1 s 2 Li – 1 s 22 s 1 Be – 1 s 22 s 2 B – 1 s 2 2 p 1 C- 1 s 2 2 p 2 Where do we start? How about with 1 s. Then just keep moving until you have placed the correct number of electrons.

Noble Gas Short-Cut Find your element on P. T. Then move all the way to the right and up one. That is your noble gas you will use as a short-cut. Then start with what period your element is in. Let’s do potassium Long way - K – 1 s 2 2 p 6 3 s 2 3 p 6 4 s 1 Short-cut - K – [Ar] 4 s 1 Electrons in Ar = 18, therefore we only need one more electron

Valence Electrons in the outermost energy level Used to determine the charge or number of bonds Both have 6 valence electrons O – [He] 2 s 2 2 p 4 8 e- 2 e- Se – [Ar] 4 s 2 3 d 10 4 p 4 34 e- 18 e- because they are both in group 16

Val e-, bonds, and charges Gp 1 gp 2 gp 13 gp 14 gp 15 gp 16 gp 17 gp 18 1 val 2 val 3 val 4 val 5 val 6 val 7 val 8 val 1 2 3 4 3 2 1 0 1+ 2+ 3+ 4+ 3 - 2 - 1 - 0 Octet Rule: Atoms want 8 valence electrons Duet rule: Atoms want 2 val e- (H, He, Li, Be, B)
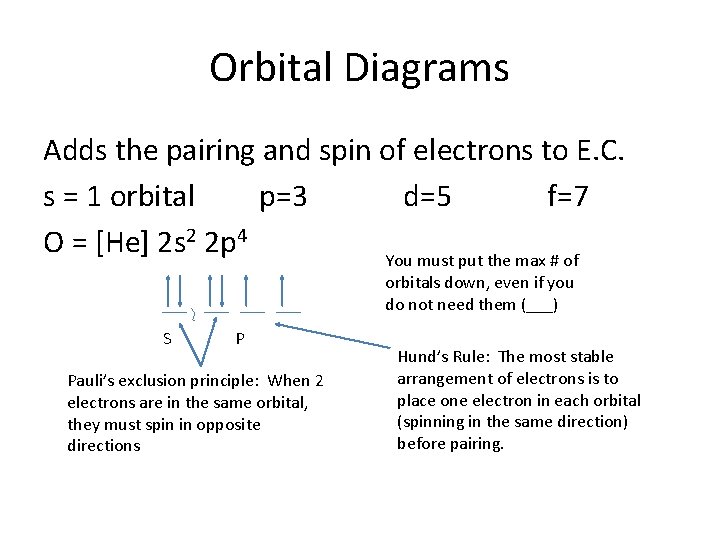
Orbital Diagrams Adds the pairing and spin of electrons to E. C. s = 1 orbital p=3 d=5 f=7 O = [He] 2 s 2 2 p 4 You must put the max # of orbitals down, even if you do not need them (___) S P Pauli’s exclusion principle: When 2 electrons are in the same orbital, they must spin in opposite directions Hund’s Rule: The most stable arrangement of electrons is to place one electron in each orbital (spinning in the same direction) before pairing.
![More examples V – 23 e- [Ar] 4 s 2 3 d 3 2 More examples V – 23 e- [Ar] 4 s 2 3 d 3 2](http://slidetodoc.com/presentation_image/95face495f0f20c3d0940d76aa340499/image-10.jpg)
More examples V – 23 e- [Ar] 4 s 2 3 d 3 2 val e- 18 e- Sb – [Kr] 5 s 2 4 d 10 5 p 3 51 e- 36 e- 5 val e-
- Slides: 10