Electron Configurations An electron configuration is the arrangement















- Slides: 23

Electron Configurations

• An electron configuration is the arrangement of electrons in an atom • A unique configuration exists for atoms of each element • The lowest energy arrangement of electrons for an element is its ground-state electron configuration.

Rules • Aufbau principle • Pauli Exclusion principle • Hund’s rule • First have to determine the energy levels, then add the electrons to the orbitals, one by one, according to the rules.

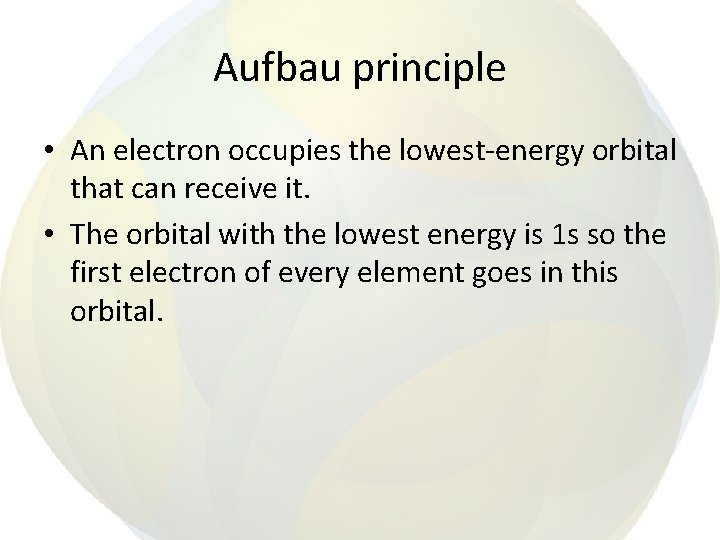
Aufbau principle • An electron occupies the lowest-energy orbital that can receive it. • The orbital with the lowest energy is 1 s so the first electron of every element goes in this orbital.

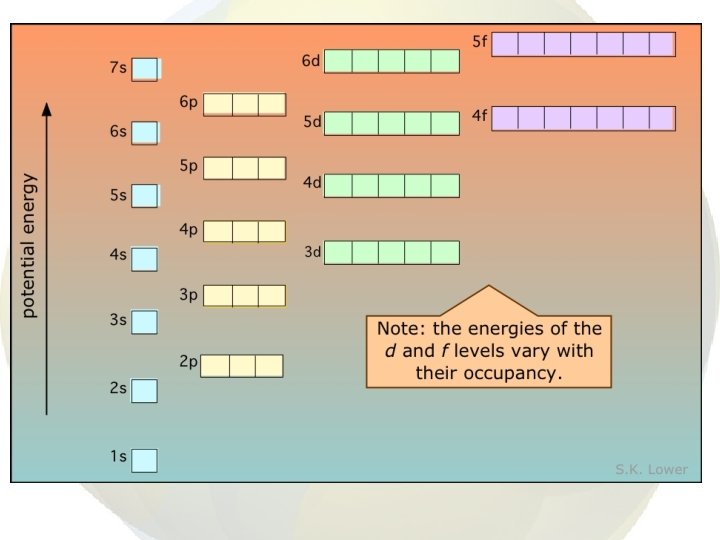
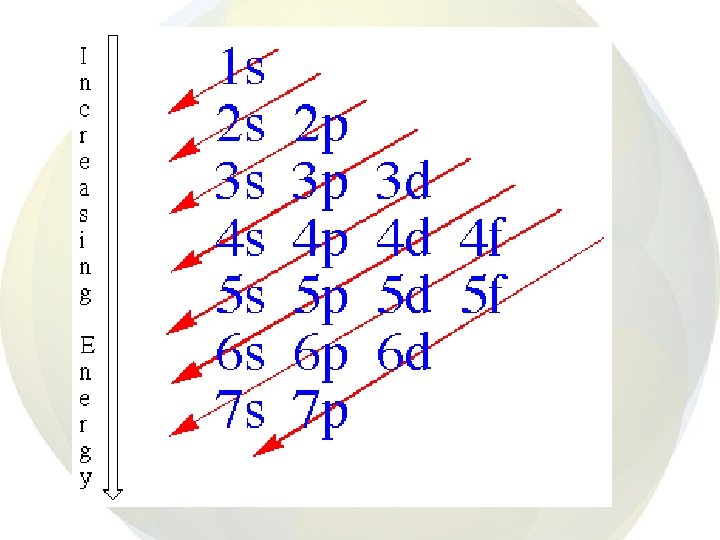
• • After 1 s is full, electrons go into the 2 s orbital Then the 2 p orbitals are filled Then 3 s and 3 p But, at this point, energies of the sublevels start overlapping.




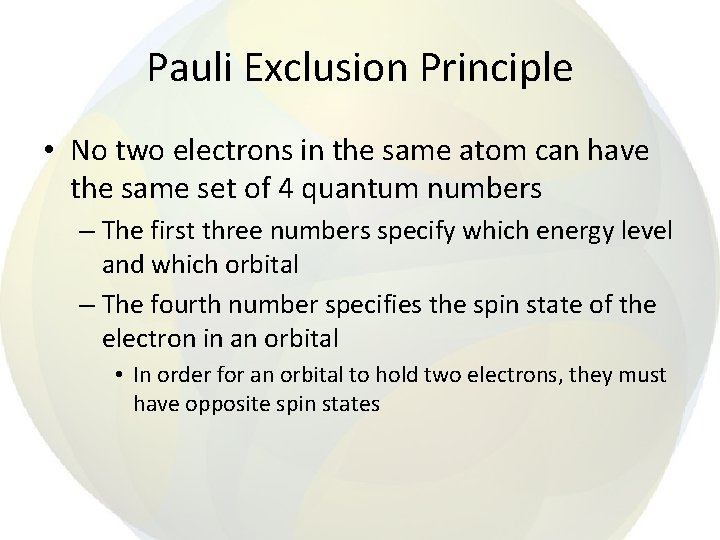
Pauli Exclusion Principle • No two electrons in the same atom can have the same set of 4 quantum numbers – The first three numbers specify which energy level and which orbital – The fourth number specifies the spin state of the electron in an orbital • In order for an orbital to hold two electrons, they must have opposite spin states

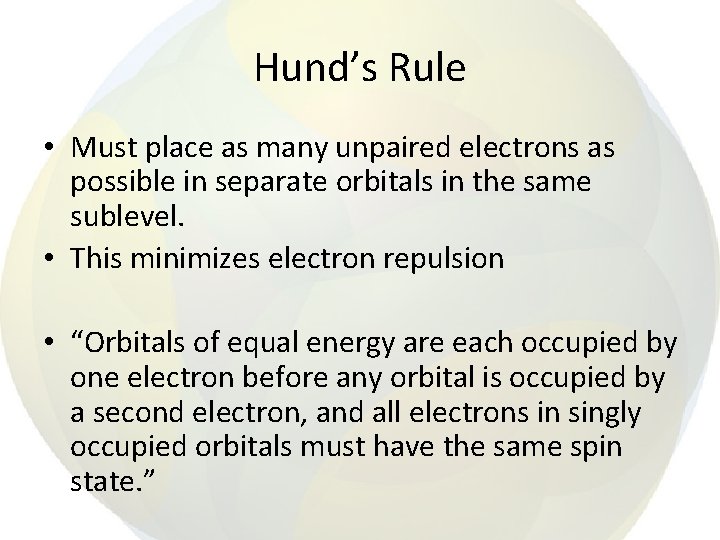
Hund’s Rule • Must place as many unpaired electrons as possible in separate orbitals in the same sublevel. • This minimizes electron repulsion • “Orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron, and all electrons in singly occupied orbitals must have the same spin state. ”

• Hund’s rule is not needed for s orbitals • In p orbitals, one electron goes in each of the three p orbitals at an energy level before any of those orbitals gets a second electron.

• We can represent electron configurations in 3 different ways – Orbital notation – Electron configuration notation – Noble gas notation

Orbital Notation • Uses lines to represent orbitals and arrows to represent electrons • The lines are labeled with the principal quantum number and sublevel letter.

• Helium • Lithium • Boron • Argon

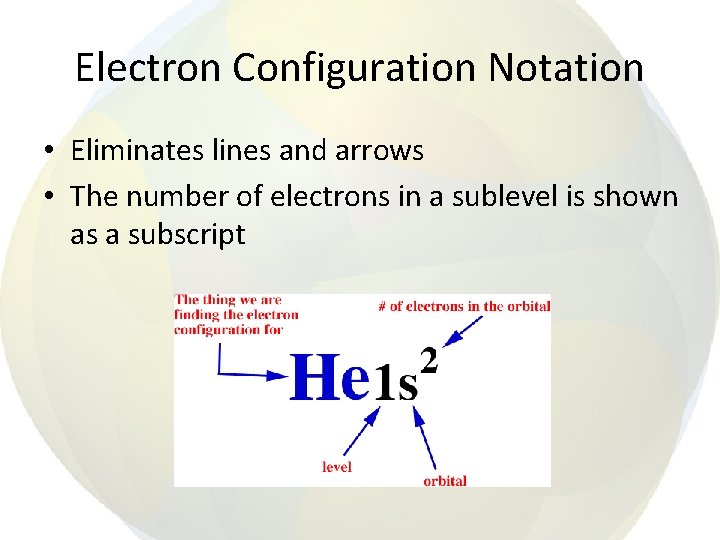
Electron Configuration Notation • Eliminates lines and arrows • The number of electrons in a sublevel is shown as a subscript

• Boron • Fluorine • Argon • Titanium • Krypton

• The highest occupied energy level is the main energy level with the highest principal quantum number that contains at least one electron • All other energy levels contain inner shell electrons

• Boron • Silicon • Selenium • Cadmium

Noble-Gas Notation • As you have seen, once you get past the 1 st two periods, orbital notation and electron configuration notation get longer and longer. • In addition, the first 10 electrons of any element in period 3 have the same configuration as a neon atom (the noble gas at the end of the 2 nd period) • This allows us to use a shorthand notation for elements in the 3 rd and higher periods.

• Sodium • Sulfur • Calcium • Nickel • Strontium

• Promethium • Seaborgium

Deviations • Starting in the 4 th period, there are some elements that have electron configurations that seem to contradict the Aufbau principle. • The first deviation is Chromium: • The actual configuration gives the lowest energy state for chromium – meaning it is more stable

• Copper • Niobium • Gold