Electron Configuration Why we study electrons Knowing the





![C. Shorthand practice Example - Germanium [Ar] 2 4 s 10 3 d 2 C. Shorthand practice Example - Germanium [Ar] 2 4 s 10 3 d 2](https://slidetodoc.com/presentation_image_h2/b5cde9e7cfecc4e454d5591b7cb92e7d/image-23.jpg)
- Slides: 25

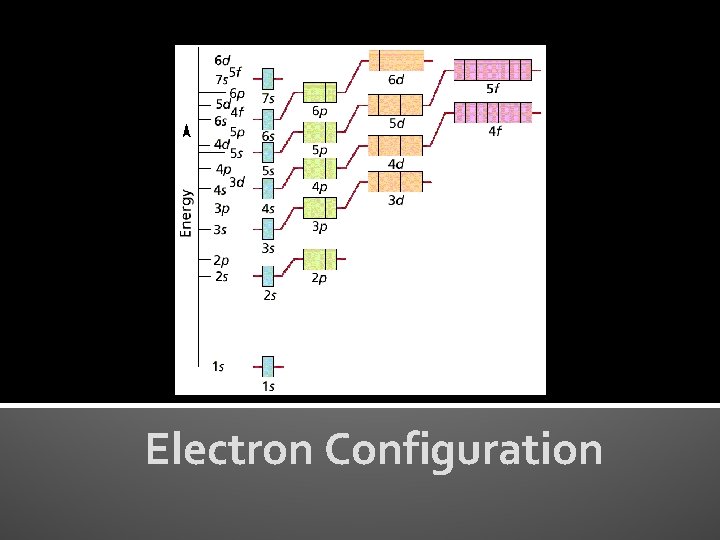
Electron Configuration

Why we study electrons Knowing the position of electrons helps us with many topics: Why chemical reactions occur Why some atoms are more stable than others Why some elements react with only certain atoms We want to know how many electrons an atom has, and where they’re located.

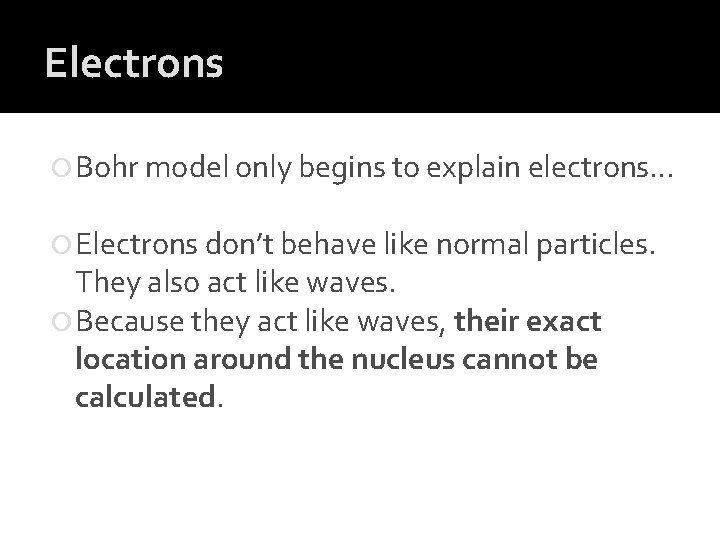
Electrons Bohr model only begins to explain electrons… Electrons don’t behave like normal particles. They also act like waves. Because they act like waves, their exact location around the nucleus cannot be calculated.

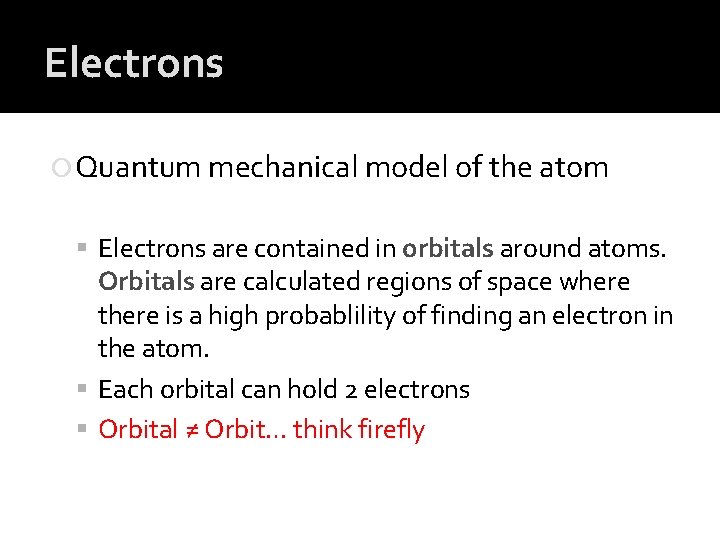
Electrons Quantum mechanical model of the atom Electrons are contained in orbitals around atoms. Orbitals are calculated regions of space where there is a high probablility of finding an electron in the atom. Each orbital can hold 2 electrons Orbital ≠ Orbit… think firefly

Quantum Numbers There are 4 quantum numbers that describe the energy and approximate position of an electron Principal Quantum Number (energy level): describes size of orbital Angular Quantum Number (subshells): describe the shape of the orbital Magnetic Quantum Number: describes the orientation in space of an orbital (x, y, z-axis) Spin: clockwise or counterclockwise spin

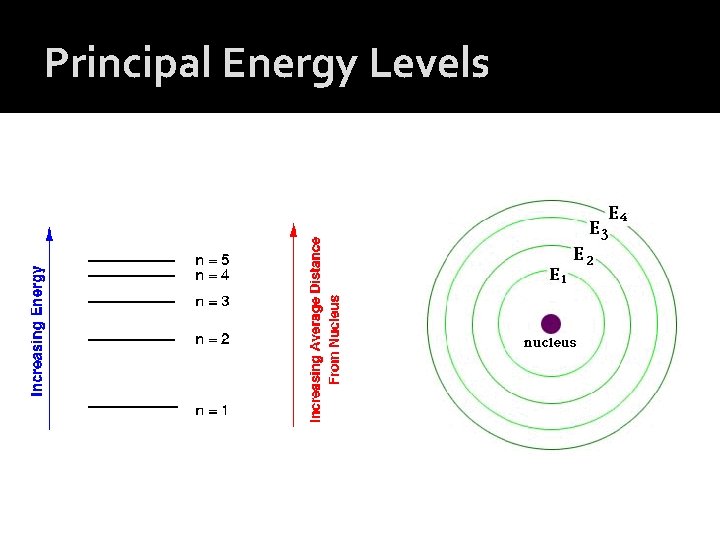
Principal Energy Levels

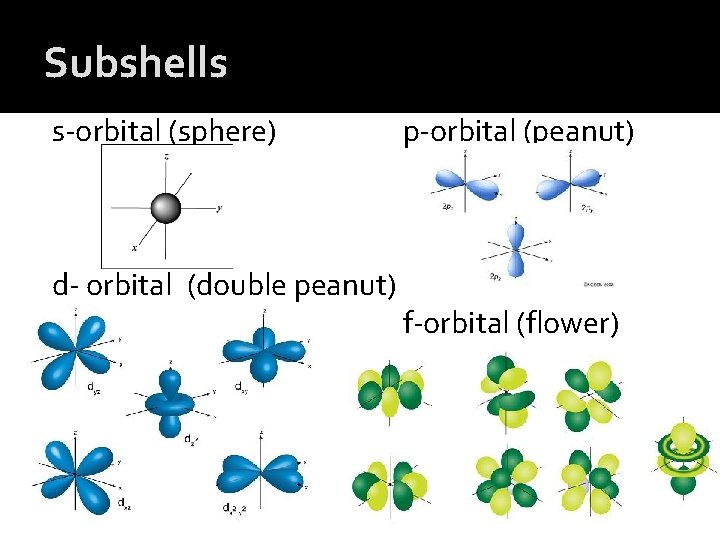
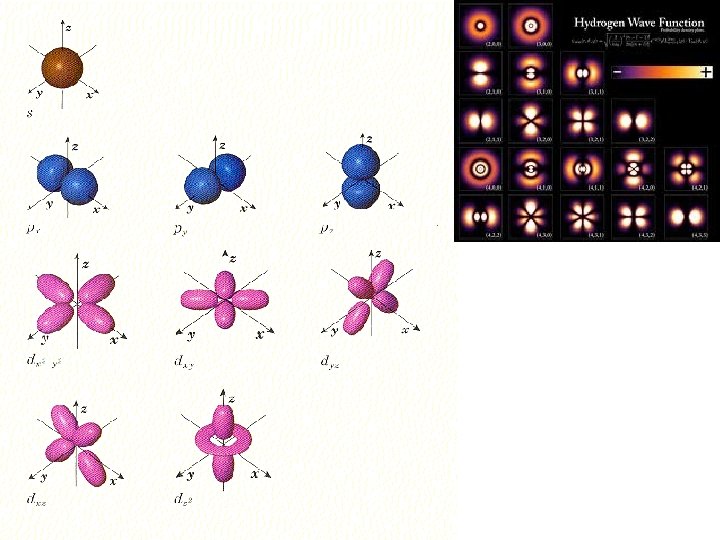
Subshells s-orbital (sphere) d- orbital (double peanut) p-orbital (peanut) f-orbital (flower)

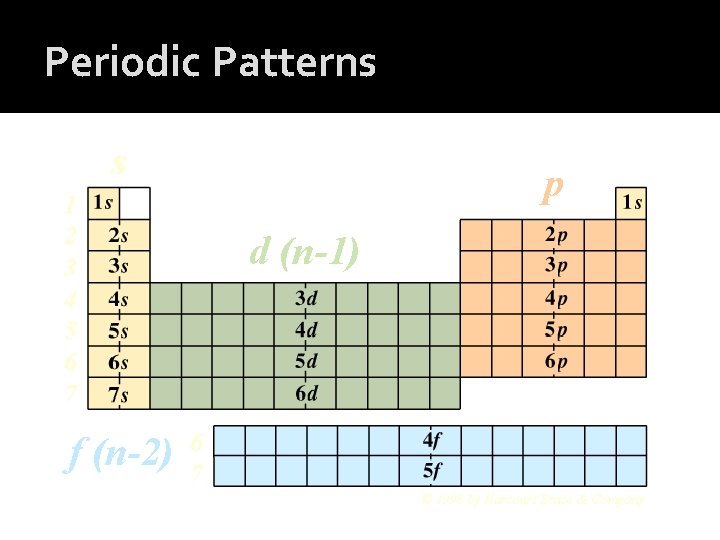
Periodic Patterns s p 1 2 3 4 5 6 7 f (n-2) d (n-1) 6 7 © 1998 by Harcourt Brace & Company

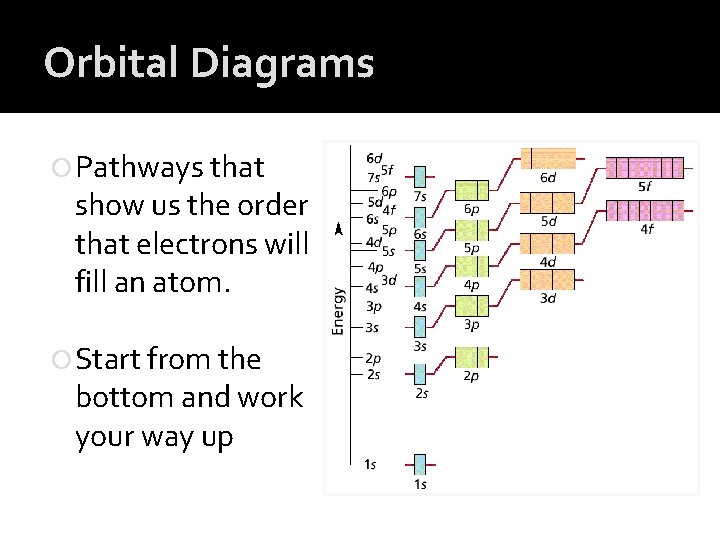
Orbital Diagrams Pathways that show us the order that electrons will fill an atom. Start from the bottom and work your way up

Orbital Diagrams When working out electron configuration, it’s important to understand orbital diagrams. Here are 3 rules that will help us understand interpret orbital diagrams.

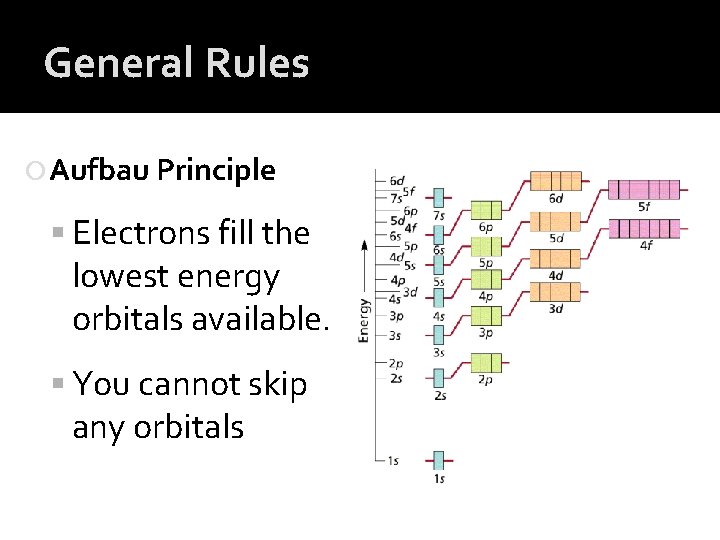
General Rules Aufbau Principle Electrons fill the lowest energy orbitals available. You cannot skip any orbitals

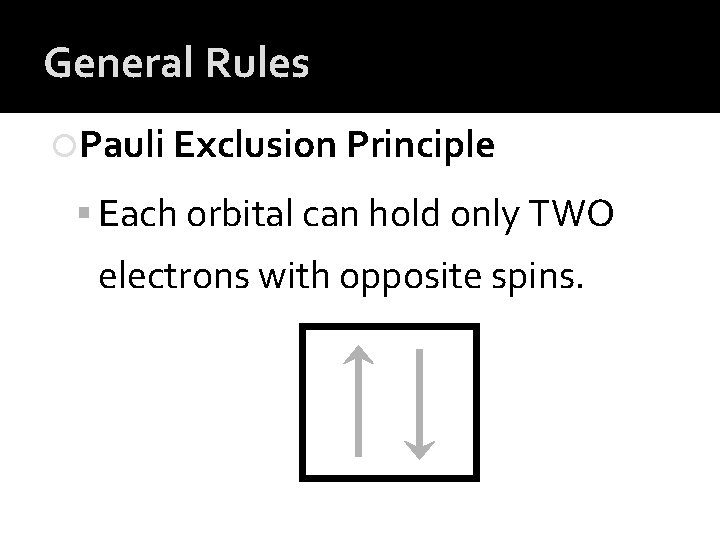
General Rules Pauli Exclusion Principle Each orbital can hold only TWO electrons with opposite spins.

General Rules Hund’s Rule Within a sublevel, place one e- per orbital with the same spin before pairing them. “Empty Seat Rule” WRONG RIGHT

A Few more things…. Each arrow you draw represents an electron. (remember the three rules!) Each orbital holds 2 electrons and has different shapes The S level has only 1 orbital= total of 2 e The P level has 3 orbitals = total of 6 e The D level has 5 orbitals = total of 10 e The F level has 7 orbitals = total of 14 e-


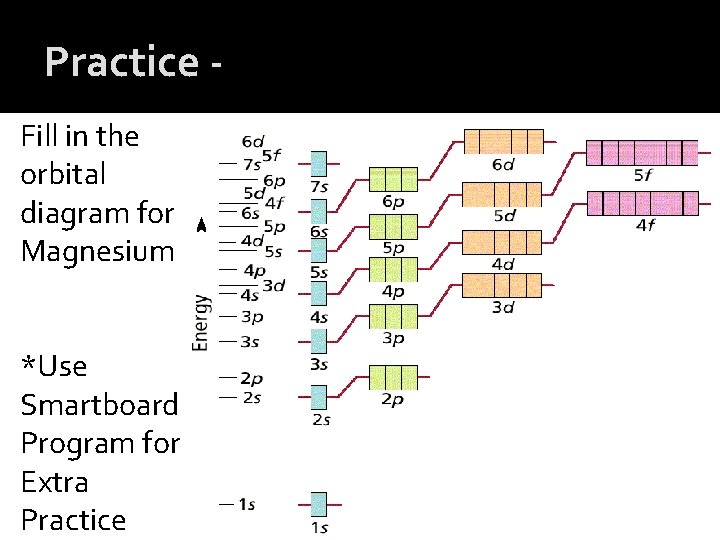
Practice Fill in the orbital diagram for Magnesium *Use Smartboard Program for Extra Practice

Practice Orbital Diagram WS Abbreviated orbital diagram (Boxes or dashes)

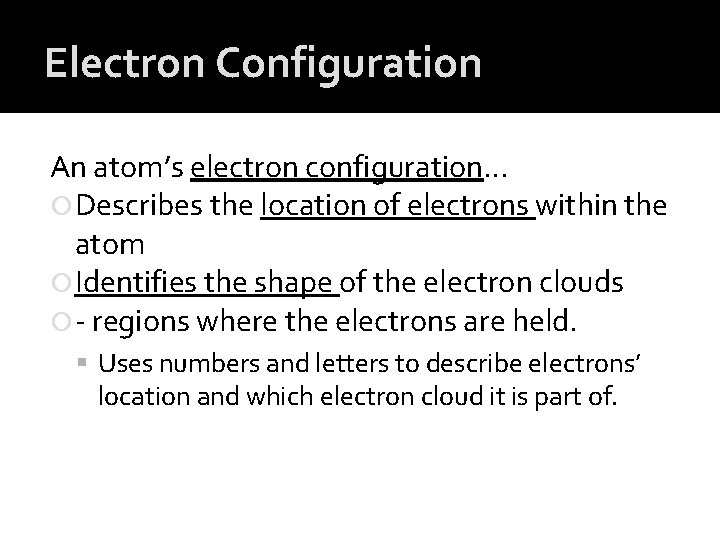
Electron Configuration An atom’s electron configuration… Describes the location of electrons within the atom Identifies the shape of the electron clouds - regions where the electrons are held. Uses numbers and letters to describe electrons’ location and which electron cloud it is part of.

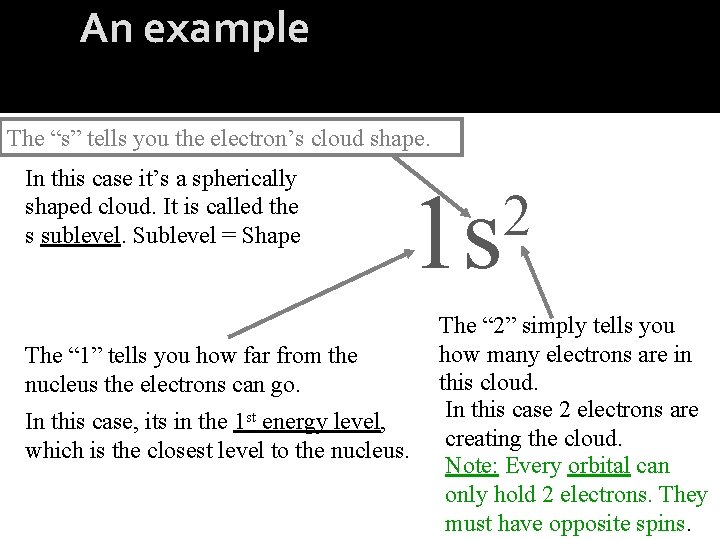
An example The “s” tells you the electron’s cloud shape. In this case it’s a spherically shaped cloud. It is called the s sublevel. Sublevel = Shape 1 s The “ 1” tells you how far from the nucleus the electrons can go. In this case, its in the 1 st energy level, which is the closest level to the nucleus. 2 The “ 2” simply tells you how many electrons are in this cloud. In this case 2 electrons are creating the cloud. Note: Every orbital can only hold 2 electrons. They must have opposite spins.

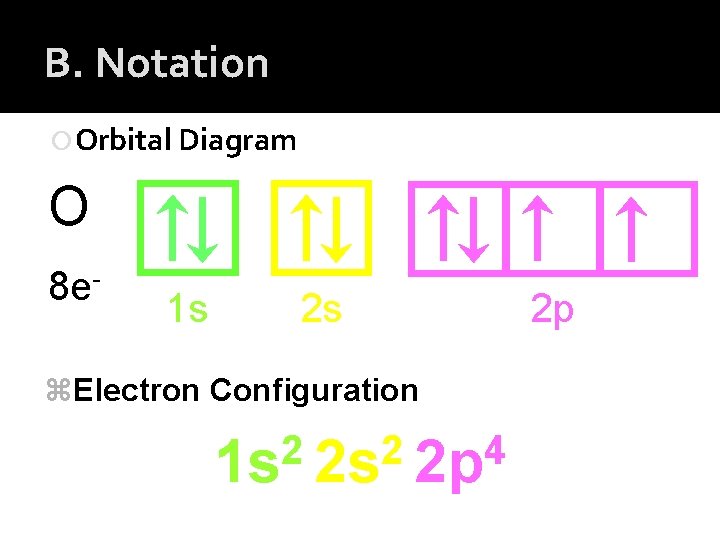
B. Notation Orbital Diagram O 8 e- 1 s 2 s z. Electron Configuration 2 2 4 1 s 2 s 2 p 2 p

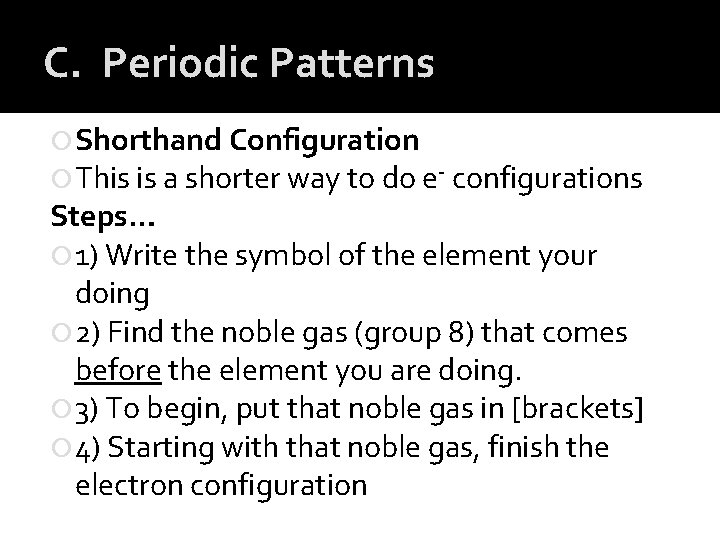
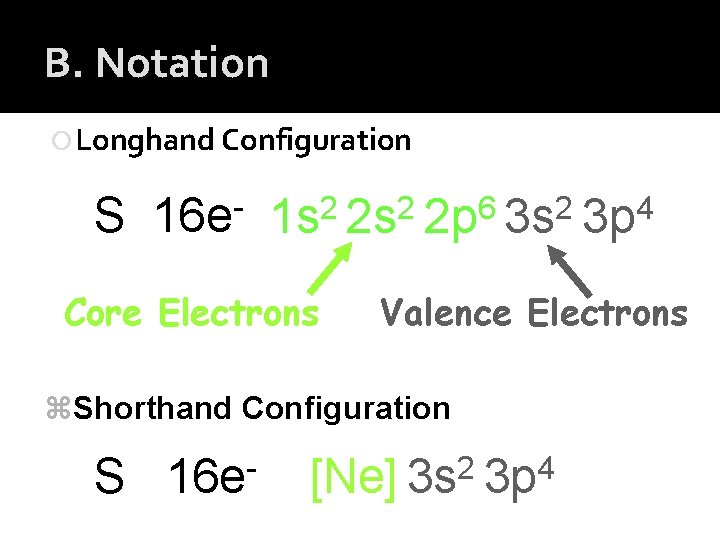
C. Periodic Patterns Shorthand Configuration This is a shorter way to do e- configurations Steps… 1) Write the symbol of the element your doing 2) Find the noble gas (group 8) that comes before the element you are doing. 3) To begin, put that noble gas in [brackets] 4) Starting with that noble gas, finish the electron configuration

B. Notation Longhand Configuration S 16 e 6 2 2 2 1 s 2 s 2 p 3 s Core Electrons Valence Electrons z. Shorthand Configuration S 16 e 4 3 p 2 4 [Ne] 3 s 3 p
![C Shorthand practice Example Germanium Ar 2 4 s 10 3 d 2 C. Shorthand practice Example - Germanium [Ar] 2 4 s 10 3 d 2](https://slidetodoc.com/presentation_image_h2/b5cde9e7cfecc4e454d5591b7cb92e7d/image-23.jpg)
C. Shorthand practice Example - Germanium [Ar] 2 4 s 10 3 d 2 4 p

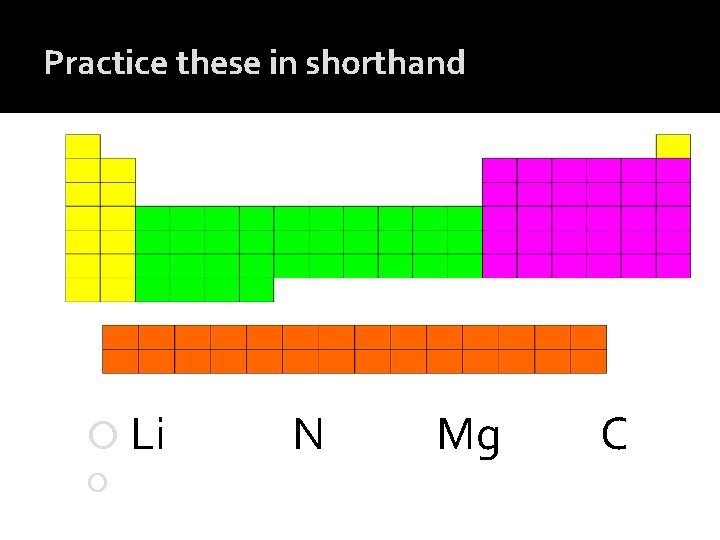
Practice these in shorthand Li N Mg C

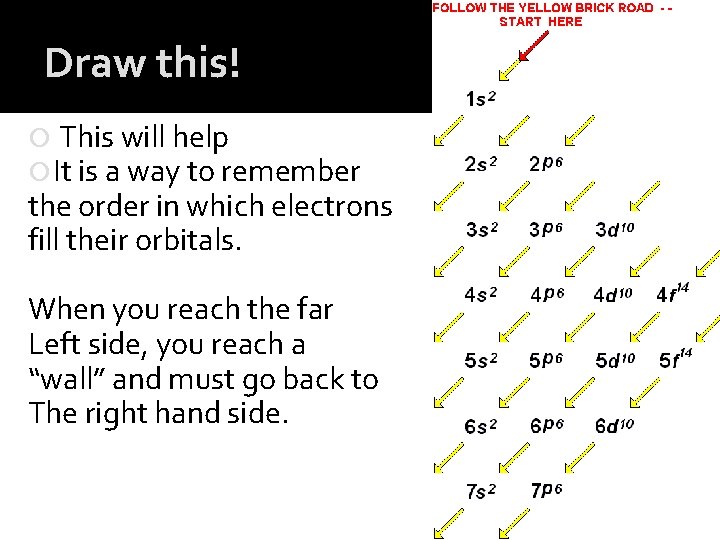
Draw this! This will help It is a way to remember the order in which electrons fill their orbitals. When you reach the far Left side, you reach a “wall” and must go back to The right hand side.