Electron Configuration When in their most stable configuration
Electron Configuration When in their most stable configuration, called the ground-state, the arrangement of electron orbitals can be organized according to three rules, or principles: The aufbau principle states that each electron occupies the lowest energy level available (electrons generally “fill in” from the nucleus outward). The Pauli exclusion principal states that the two electrons found in an orbital have opposite “spins” (not really a spin, but close enough for our class), represented by “up” and “down” arrows: ↑↓ Hund’s rule states that single electrons of identical spin must occupy each equal-energy orbital before additional electrons of opposite spin can occupy the same orbital. In a p-energy level, for example, the first 3 electrons occupy ↑ ↑ ↑ , not ↑↓ ↑.
Aufbau Principle All orbitals related to an energy sublevel are of equal energy (px, py, and pz are all of equal energy). Different energy sublevels within a principle energy level have different energies (the 2 p sublevel has higher energy than the 2 s sublevel). In order of increasing energy within a principle energy level, the sublevel sequence is s, p, d, and f. There can be overlap of energy amounts between orbitals of different primary energy levels. The 4 s energy sublevel has less energy than the 3 d energy sublevel, even though n = 4 is farther from the nucleus than n = 3.
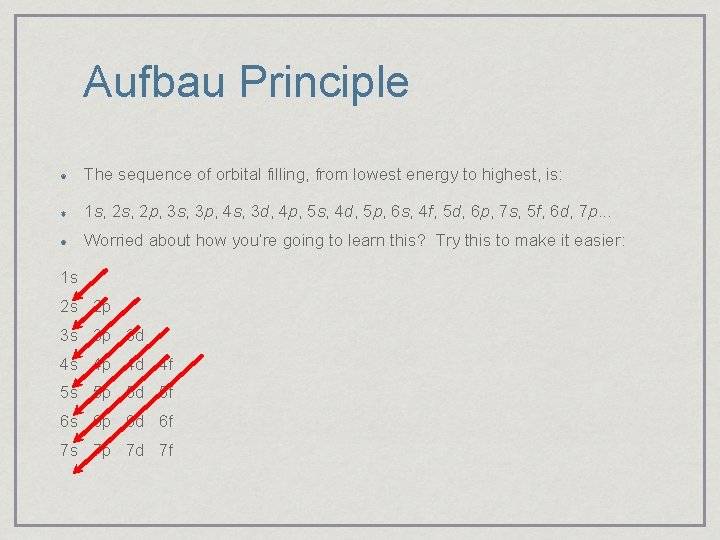
Aufbau Principle The sequence of orbital filling, from lowest energy to highest, is: 1 s, 2 p, 3 s, 3 p, 4 s, 3 d, 4 p, 5 s, 4 d, 5 p, 6 s, 4 f, 5 d, 6 p, 7 s, 5 f, 6 d, 7 p. . . Worried about how you’re going to learn this? Try this to make it easier: 1 s 2 s 2 p 3 s 3 p 3 d 4 s 4 p 4 d 4 f 5 s 5 p 5 d 5 f 6 s 6 p 6 d 6 f 7 s 7 p 7 d 7 f
Electron Configuration 1 s, 2 p, 3 s, 3 p, 4 s, 3 d, 4 p, 5 s, 4 d, 5 p, 6 s, 4 f, 5 d, 6 p, 7 s, 5 f, 6 d, 7 p. . . Oxygen has 8 electrons. According to the aufbau principle: - 2 are found in the 1 s orbital, - 2 are found in the 2 s orbital, and - 4 are found in the 2 p orbitals (px, py, and pz) According to the Pauli exclusion principle, the two electrons in 1 s have opposite “spins” (as do the two electrons in 2 s). According to Hund’s rule, the 2 px, 2 py, and 2 pz orbitals each get one of the four remaining electrons, and all three of these electrons “spin” in the same direction. The fourth electron completes one of the orbitals (it doesn’t matter which one), and “spins” in the opposite direction.
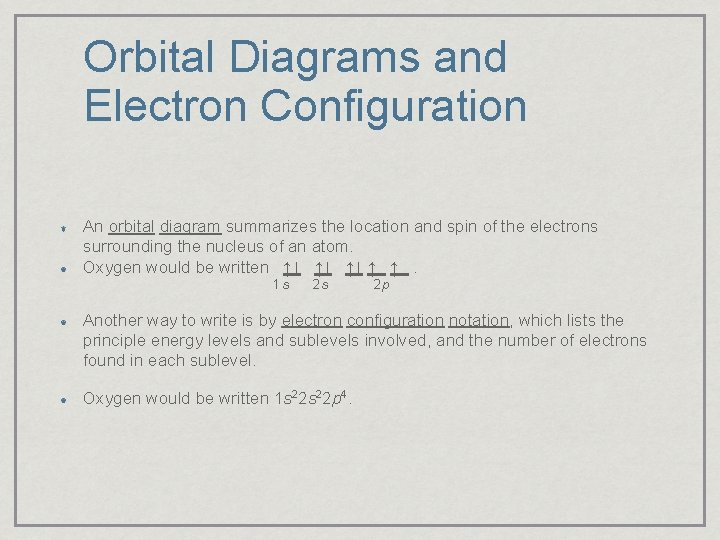
Orbital Diagrams and Electron Configuration An orbital diagram summarizes the location and spin of the electrons surrounding the nucleus of an atom. Oxygen would be written ↑↓ ↑↓ ↑↓ ↑ ↑. 1 s 2 s 2 p Another way to write is by electron configuration notation, which lists the principle energy levels and sublevels involved, and the number of electrons found in each sublevel. Oxygen would be written 1 s 22 p 4.
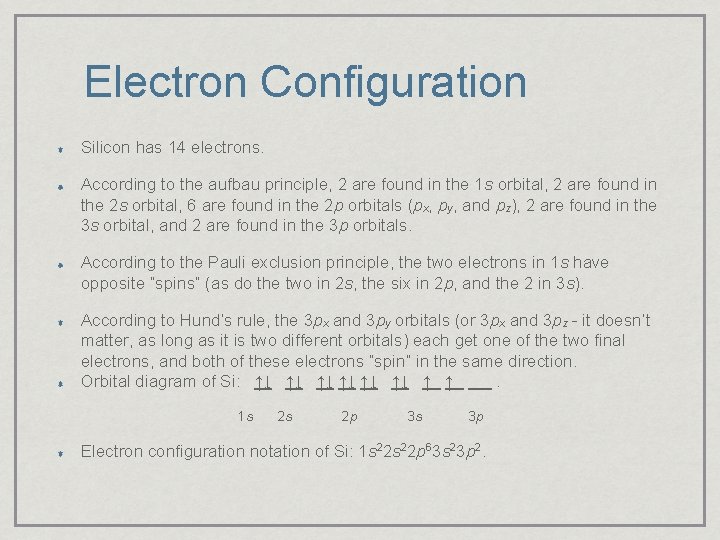
Electron Configuration Silicon has 14 electrons. According to the aufbau principle, 2 are found in the 1 s orbital, 2 are found in the 2 s orbital, 6 are found in the 2 p orbitals (px, py, and pz), 2 are found in the 3 s orbital, and 2 are found in the 3 p orbitals. According to the Pauli exclusion principle, the two electrons in 1 s have opposite “spins” (as do the two in 2 s, the six in 2 p, and the 2 in 3 s). According to Hund’s rule, the 3 px and 3 py orbitals (or 3 px and 3 pz - it doesn’t matter, as long as it is two different orbitals) each get one of the two final electrons, and both of these electrons “spin” in the same direction. Orbital diagram of Si: ↑↓ ↑↓ ↑↓ ↑ ↑. 1 s 2 s 2 p 3 s 3 p Electron configuration notation of Si: 1 s 22 p 63 s 23 p 2.
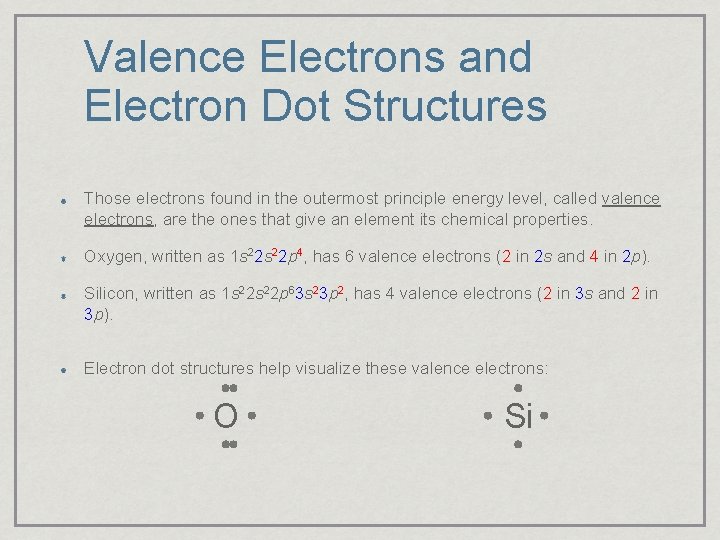
Valence Electrons and Electron Dot Structures Those electrons found in the outermost principle energy level, called valence electrons, are the ones that give an element its chemical properties. Oxygen, written as 1 s 22 p 4, has 6 valence electrons (2 in 2 s and 4 in 2 p). Silicon, written as 1 s 22 p 63 s 23 p 2, has 4 valence electrons (2 in 3 s and 2 in 3 p). Electron dot structures help visualize these valence electrons: O Si
- Slides: 7