Electron Configuration Quantum States and Orbitals Quantum state
Electron Configuration
Quantum States and Orbitals • Quantum state = a specific combination of values of variables such as energy and position that is allowed by quantum theory. • We group electrons into different quantum states to describe their electron arrangement.
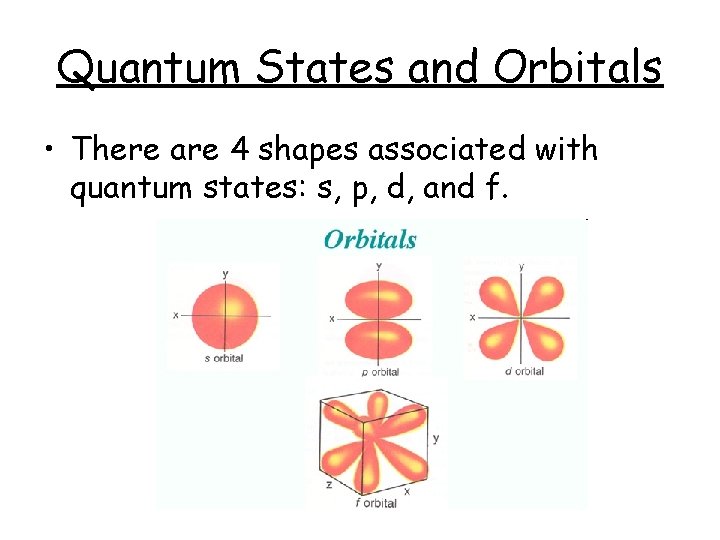
Quantum States and Orbitals • There are 4 shapes associated with quantum states: s, p, d, and f.
Orbitals • Orbitals hold electrons, and show the area in which the electrons can be found. • “s” orbitals can hold 2 electrons • “p” orbitals can hold 6 electrons • “d” orbitals can hold 10 electrons • “f” orbitals can hold 14 electrons
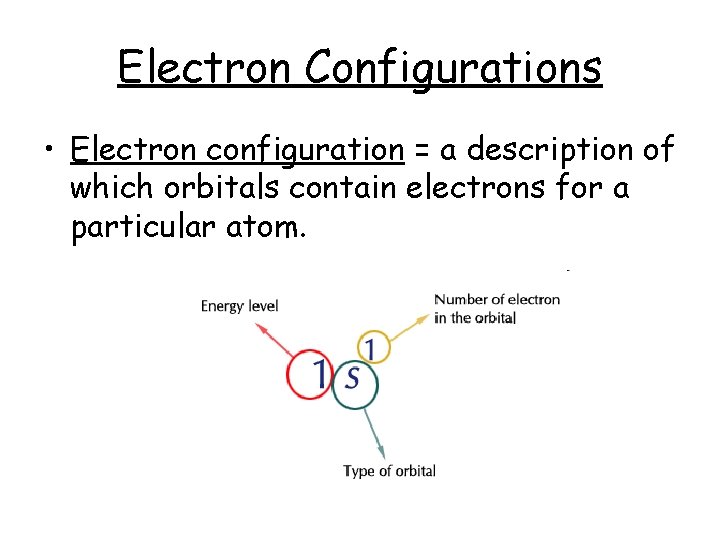
Electron Configurations • Electron configuration = a description of which orbitals contain electrons for a particular atom.
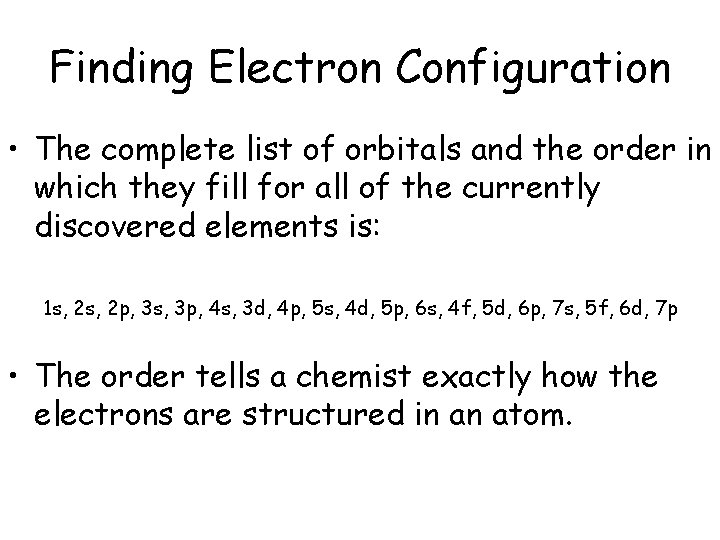
Finding Electron Configuration • The complete list of orbitals and the order in which they fill for all of the currently discovered elements is: 1 s, 2 p, 3 s, 3 p, 4 s, 3 d, 4 p, 5 s, 4 d, 5 p, 6 s, 4 f, 5 d, 6 p, 7 s, 5 f, 6 d, 7 p • The order tells a chemist exactly how the electrons are structured in an atom.

How to find electron configuration:
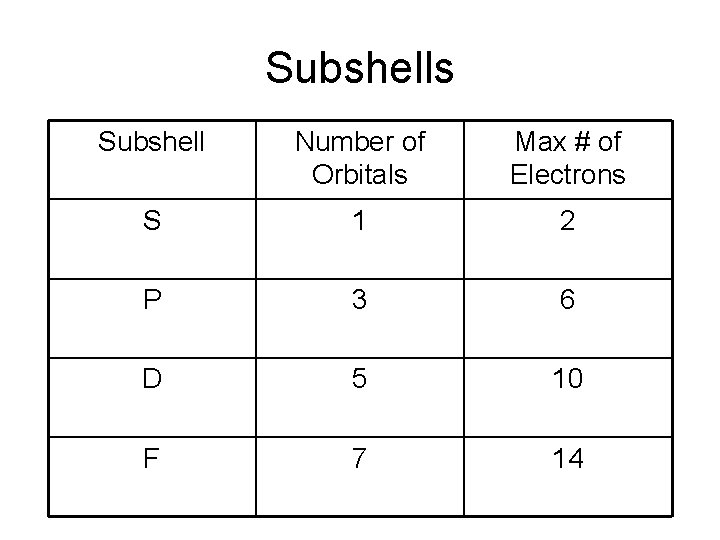
Subshells Subshell Number of Orbitals Max # of Electrons S 1 2 P 3 6 D 5 10 F 7 14
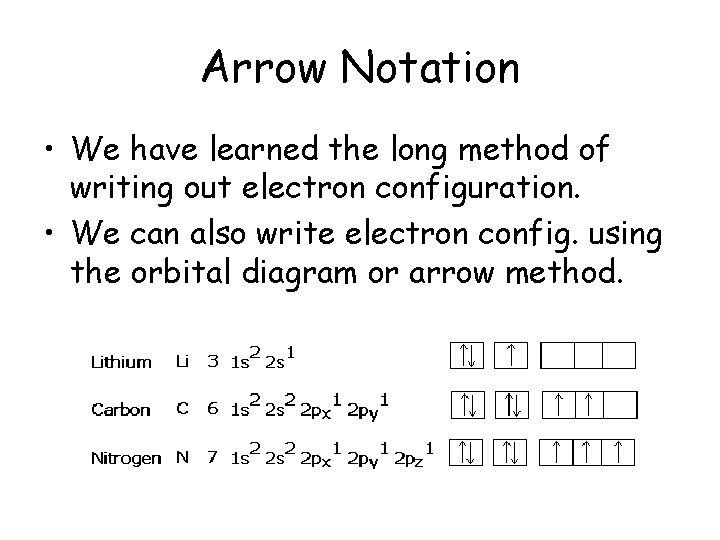
Arrow Notation • We have learned the long method of writing out electron configuration. • We can also write electron config. using the orbital diagram or arrow method.
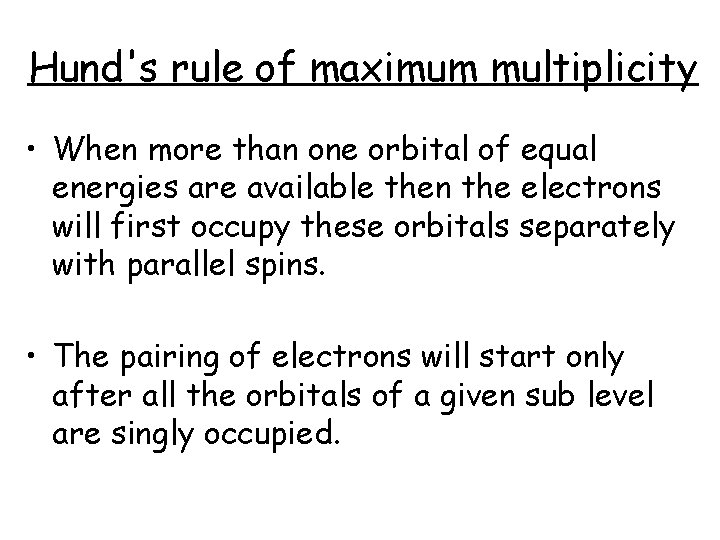
Hund's rule of maximum multiplicity • When more than one orbital of equal energies are available then the electrons will first occupy these orbitals separately with parallel spins. • The pairing of electrons will start only after all the orbitals of a given sub level are singly occupied.
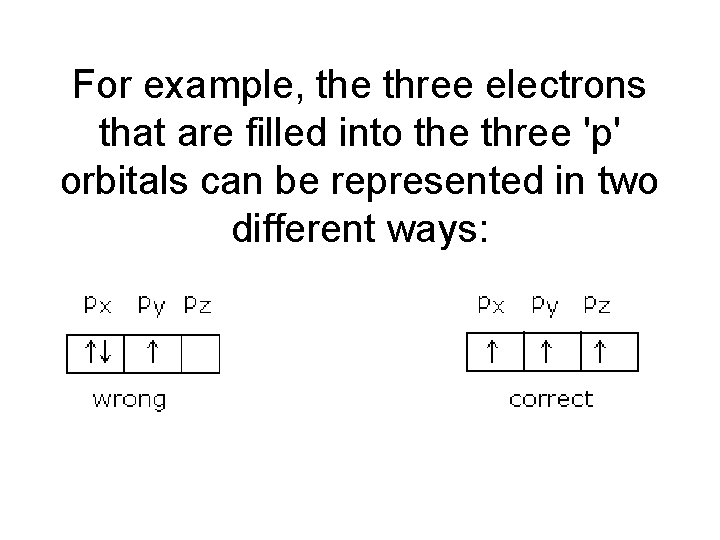
For example, the three electrons that are filled into the three 'p' orbitals can be represented in two different ways:
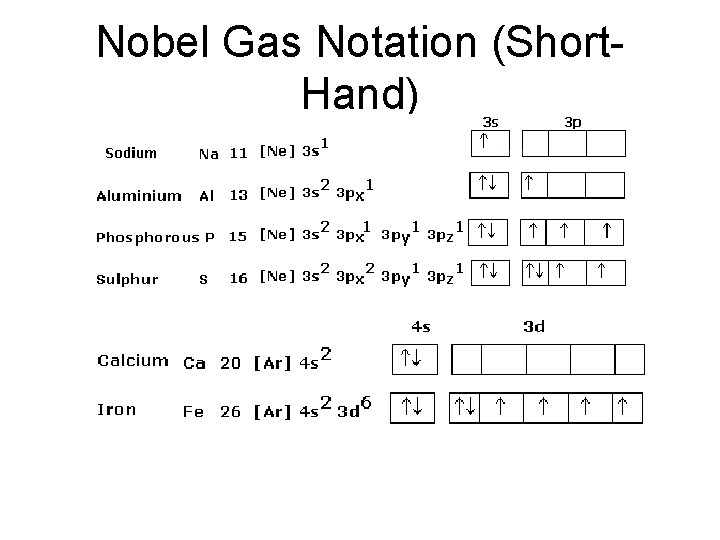
Nobel Gas Notation (Short. Hand)
- Slides: 13