Electron Configuration Orbitals 1 s 22 p 63
Electron Configuration & Orbitals 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 24 d 105 p 66 s 24 f 145 d 106 p 6…
Orbital Shapes Bohr model shows electrons travelling a 2 dimensional circular path Quantum Mechanical Model shows electrons in a 3 -dimensional region of space
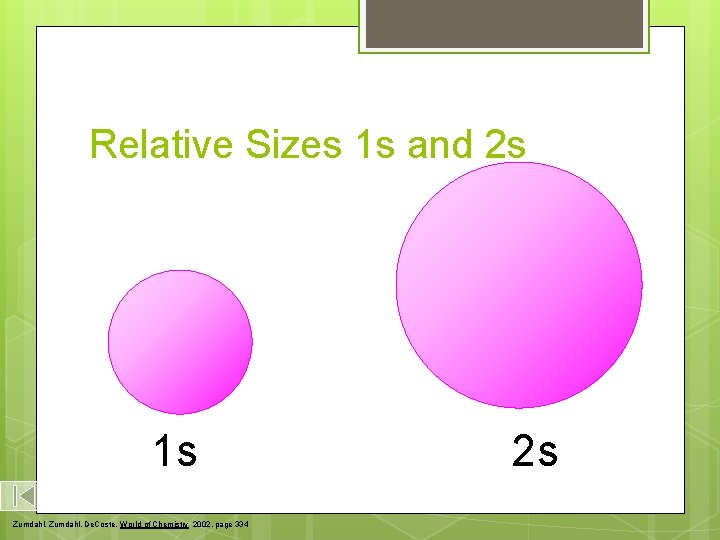
Relative Sizes 1 s and 2 s 1 s Zumdahl, De. Coste, World of Chemistry 2002, page 334 2 s
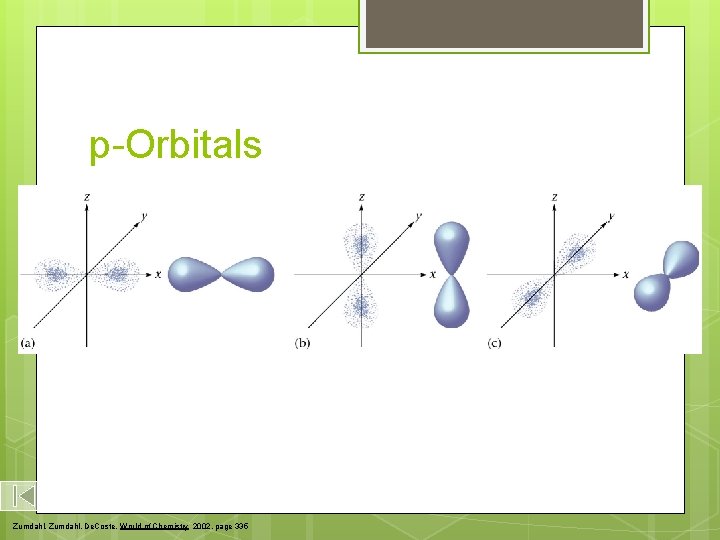
p-Orbitals Zumdahl, De. Coste, World of Chemistry 2002, page 335
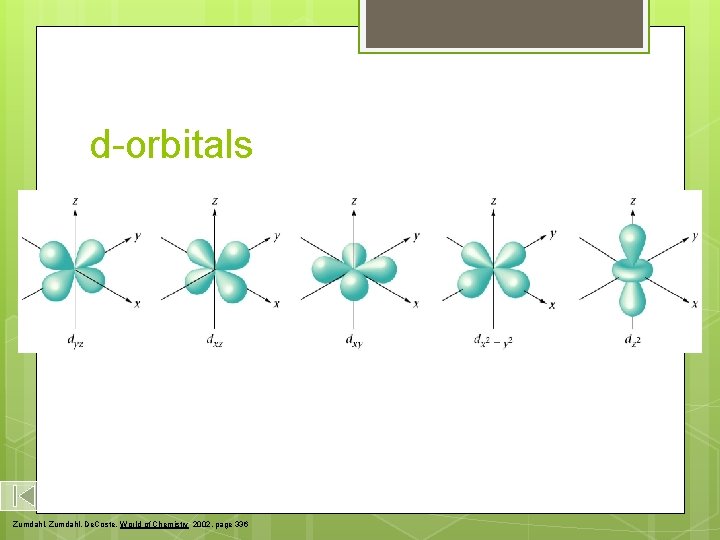
d-orbitals Zumdahl, De. Coste, World of Chemistry 2002, page 336
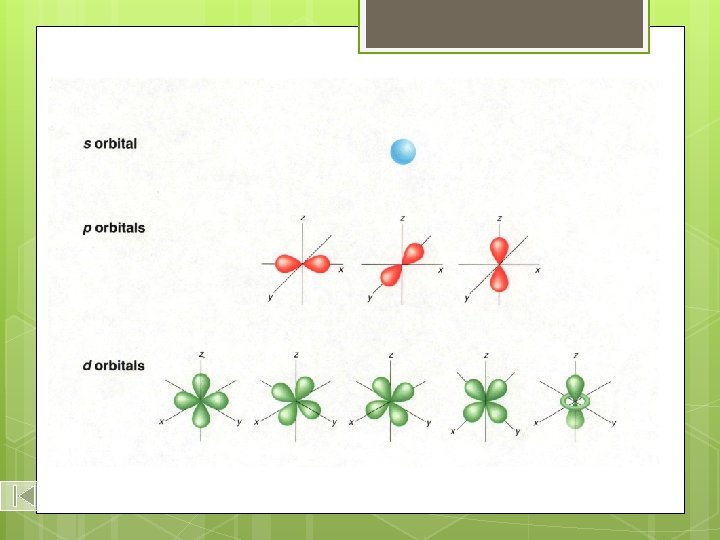
Shapes of s, p, and d-Orbitals
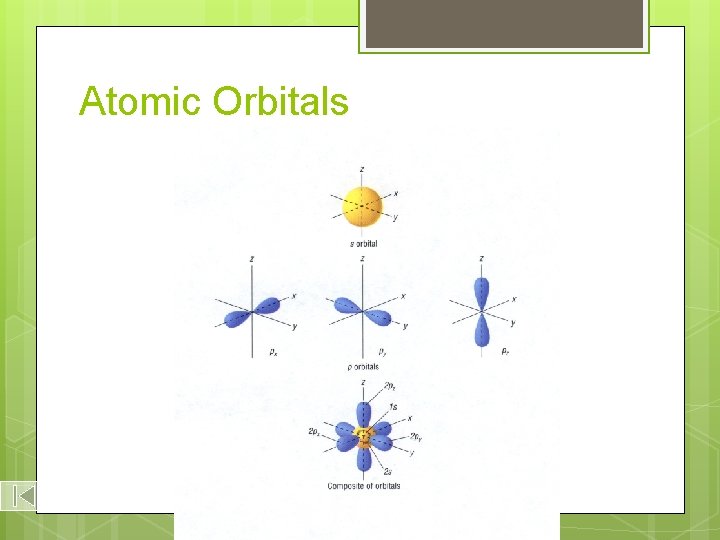
Atomic Orbitals
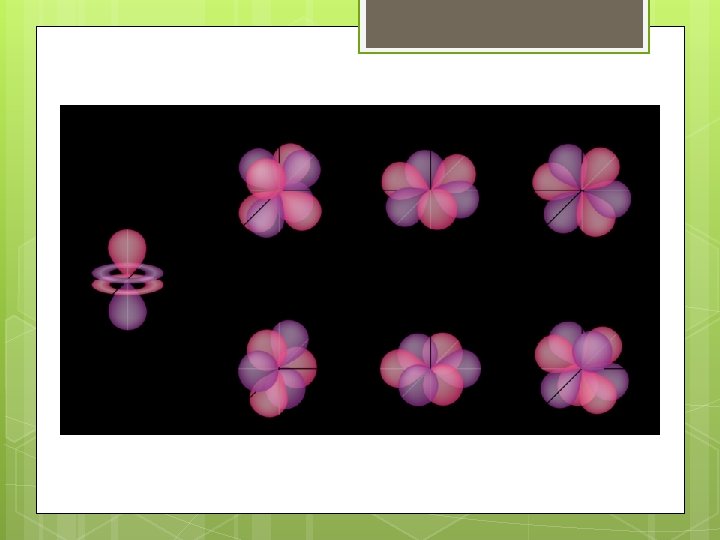
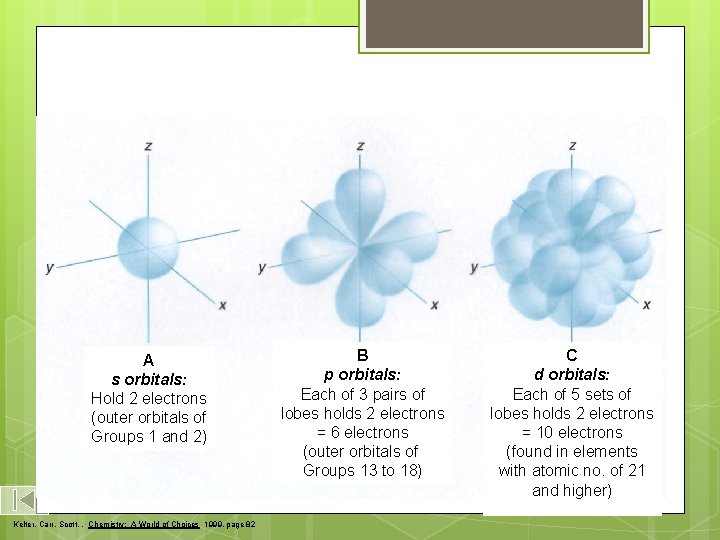
s, p, and d-orbitals A s orbitals: Hold 2 electrons (outer orbitals of Groups 1 and 2) Kelter, Carr, Scott, , Chemistry: A World of Choices 1999, page 82 B p orbitals: Each of 3 pairs of lobes holds 2 electrons = 6 electrons (outer orbitals of Groups 13 to 18) C d orbitals: Each of 5 sets of lobes holds 2 electrons = 10 electrons (found in elements with atomic no. of 21 and higher)
Say Goodbye to the Bohr Model…
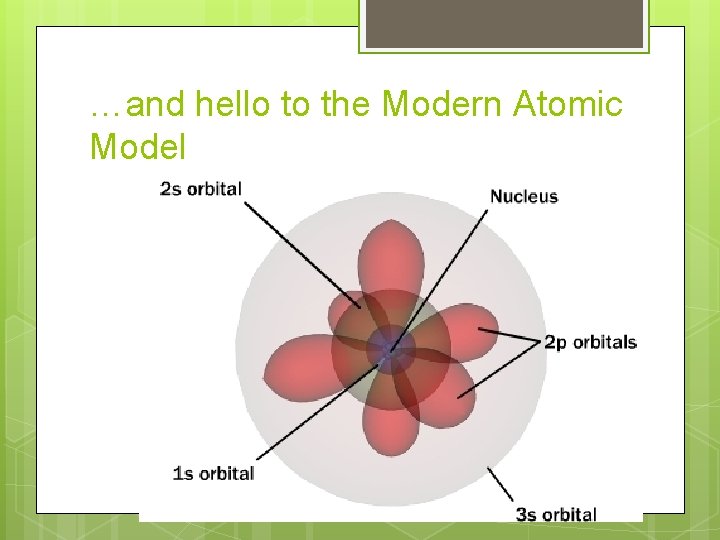
…and hello to the Modern Atomic Model

Electron Configuration Use a symbol that contains 3 pieces of information n = the number of the principal quantum shell l = the letter the designates the orbital type superscript number = designate the number of electrons in that subshell Ex: 2 p 4 Means 4 electrons in a p subshell with a principal quantum number of 2
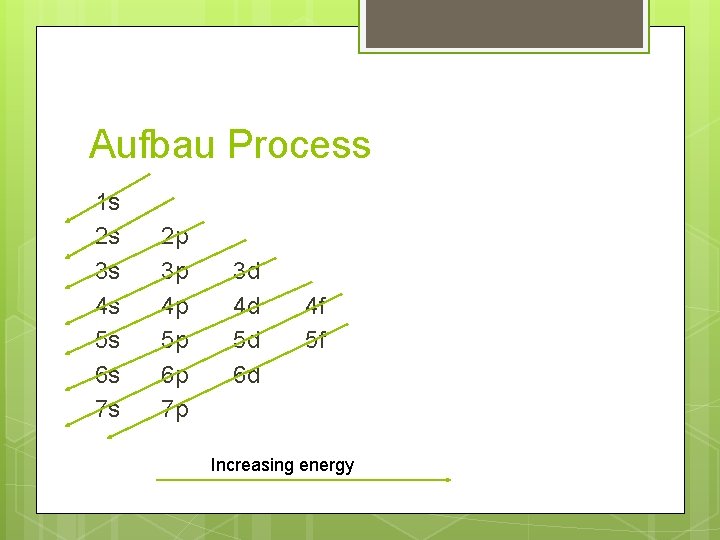
Aufbau Process 1 s 2 s 3 s 4 s 5 s 6 s 7 s 2 p 3 p 4 p 5 p 6 p 7 p 3 d 4 d 5 d 6 d 4 f 5 f Increasing energy

Filling Rules for Electron Orbitals Aufbau Principle: Electrons are added one at a time to the lowest energy orbitals available until all the electrons of the atom have been accounted for. Pauli Exclusion Principle: An orbital can hold a maximum of two electrons. To occupy the same orbital, two electrons must spin in opposite directions. Hund’s Rule: Electrons occupy equal-energy orbitals so that a maximum number of unpaired electrons results. *Aufbau is German for “building up”
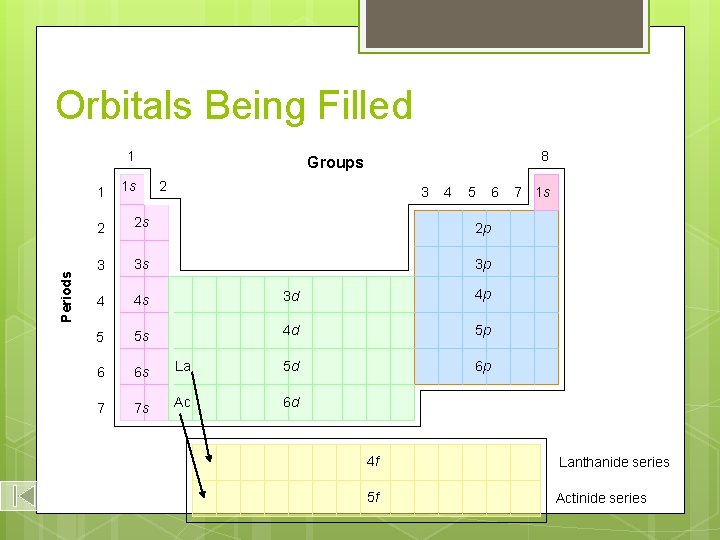
Orbitals Being Filled 1 Periods 1 1 s 8 Groups 2 3 4 5 2 2 s 2 p 3 3 s 3 p 4 4 s 3 d 4 p 5 5 s 4 d 5 p 6 6 s La 5 d 6 p 7 7 s Ac 6 d 6 7 1 s 4 f Lanthanide series 5 f Actinide series

Aligning Electron Configurations With the Periodic Table

Pauli Exclusion Principle The Pauli Exclusion Principle says that electrons in the same orbital must have opposite spins. 1 s
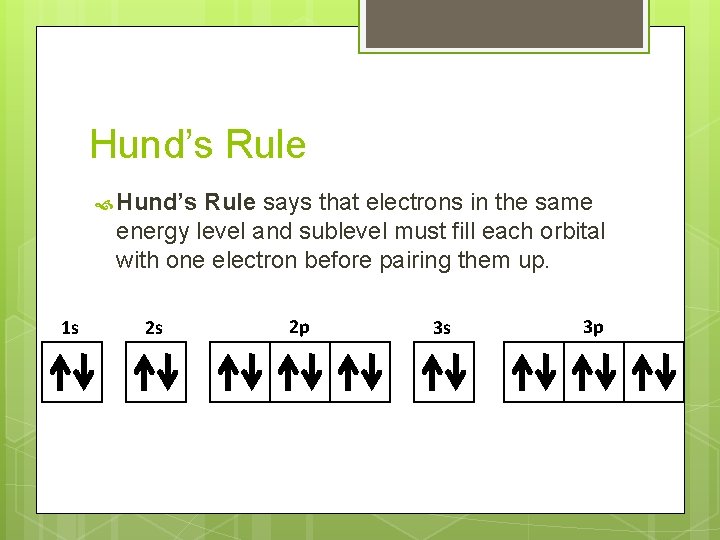
Hund’s Rule says that electrons in the same energy level and sublevel must fill each orbital with one electron before pairing them up. 1 s 2 s 2 p 3 s 3 p
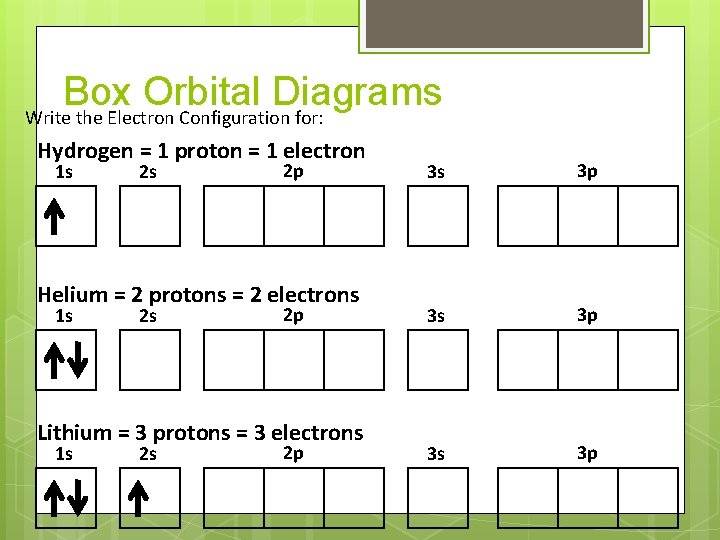
Box Orbital Diagrams Write the Electron Configuration for: Hydrogen = 1 proton = 1 electron 1 s 2 s 2 p Helium = 2 protons = 2 electrons 1 s 2 s 2 p Lithium = 3 protons = 3 electrons 1 s 2 s 2 p 3 s 3 p
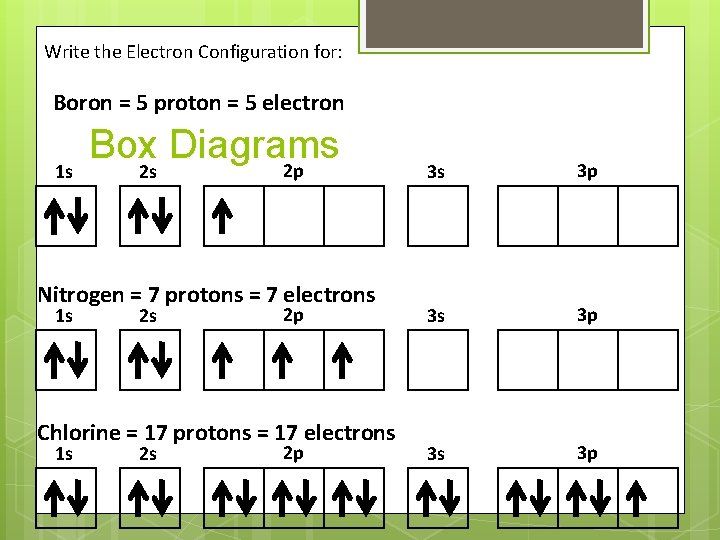
Write the Electron Configuration for: Boron = 5 proton = 5 electron 1 s Box Diagrams 2 p 2 s Nitrogen = 7 protons = 7 electrons 1 s 2 s 2 p Chlorine = 17 protons = 17 electrons 1 s 2 s 2 p 3 s 3 p
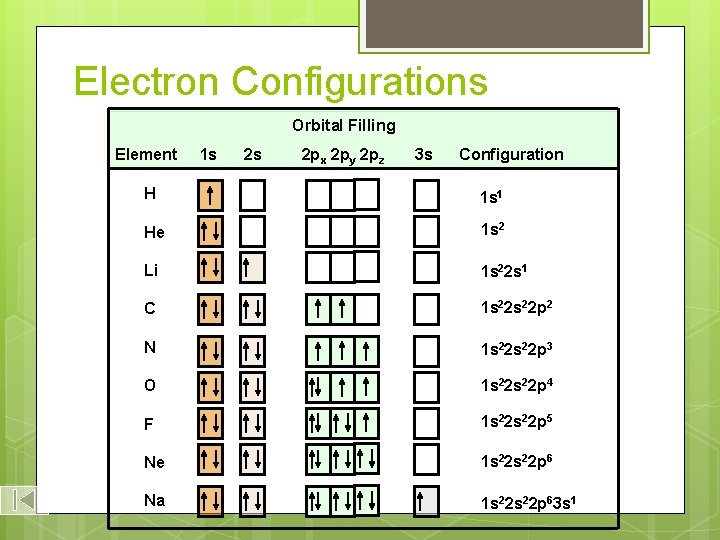
Electron Configurations Orbital Filling Element 1 s 2 s 2 px 2 py 2 pz 3 s Electron Configuration H 1 s 1 He 1 s 2 Li 1 s 22 s 1 C 1 s 22 p 2 N 1 s 22 p 3 O 1 s 22 p 4 F 1 s 22 p 5 Ne 1 s 22 p 6 Na 1 s 22 p 63 s 1
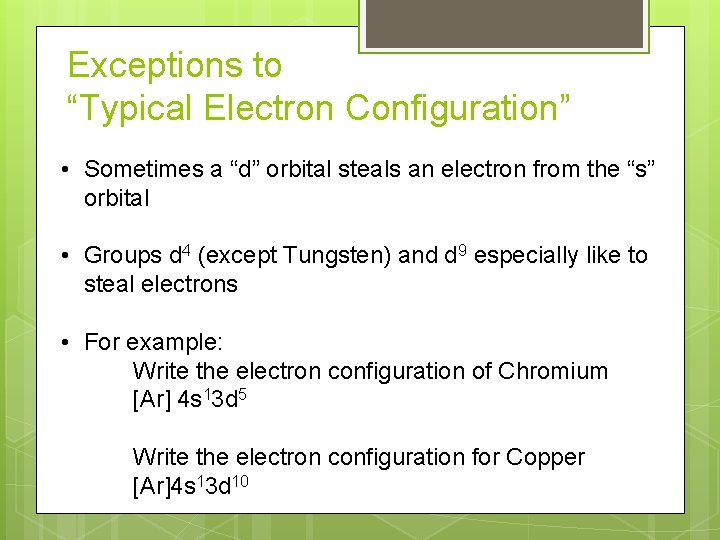
Exceptions to “Typical Electron Configuration” • Sometimes a “d” orbital steals an electron from the “s” orbital • Groups d 4 (except Tungsten) and d 9 especially like to steal electrons • For example: Write the electron configuration of Chromium [Ar] 4 s 13 d 5 Write the electron configuration for Copper [Ar]4 s 13 d 10

What happens to e- when there are ions? • Simply determine if electrons are lost or gained and then write electron configuration or orbital diagram appropriately • For example: What is the electron configuration of O 2 -? [He] 2 s 22 p 6 What is the electron configuration of Al 3+? [He] 2 s 22 p 6
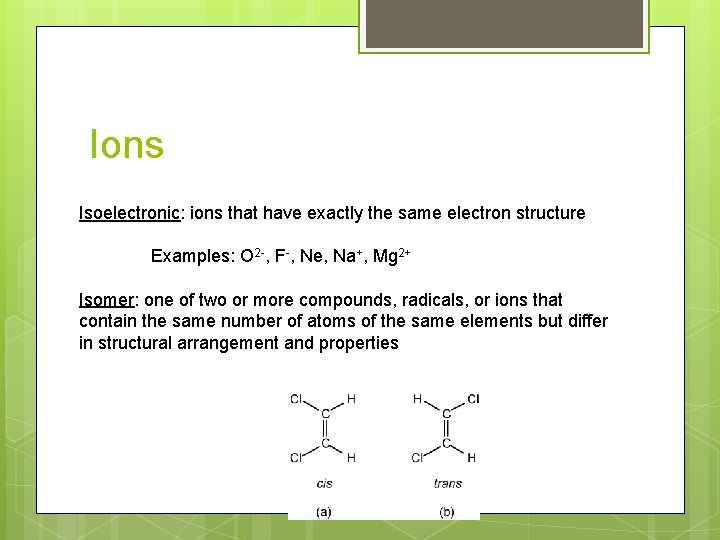
Ions Isoelectronic: ions that have exactly the same electron structure Examples: O 2 -, F-, Ne, Na+, Mg 2+ Isomer: one of two or more compounds, radicals, or ions that contain the same number of atoms of the same elements but differ in structural arrangement and properties
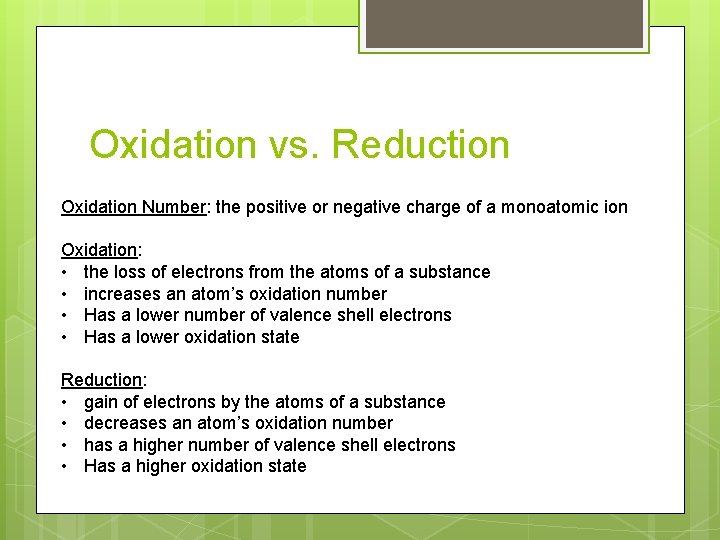
Oxidation vs. Reduction Oxidation Number: the positive or negative charge of a monoatomic ion Oxidation: • the loss of electrons from the atoms of a substance • increases an atom’s oxidation number • Has a lower number of valence shell electrons • Has a lower oxidation state Reduction: • gain of electrons by the atoms of a substance • decreases an atom’s oxidation number • has a higher number of valence shell electrons • Has a higher oxidation state
- Slides: 25