Electron Configuration Orbitals 1 s 22 p 63
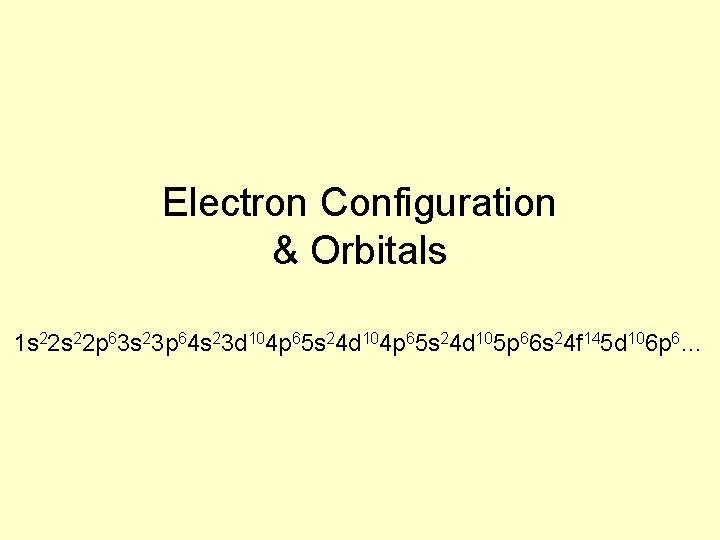
Electron Configuration & Orbitals 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 24 d 105 p 66 s 24 f 145 d 106 p 6…
Bohr’s Model • Bohr’s atomic model of electrons in orbits with specific levels of energy was only partly correct. • The idea of electron shells is not quite sufficient to explain the behaviour of atoms. • We need to add the concept of orbitals and quantum numbers for elements with multiple electrons.
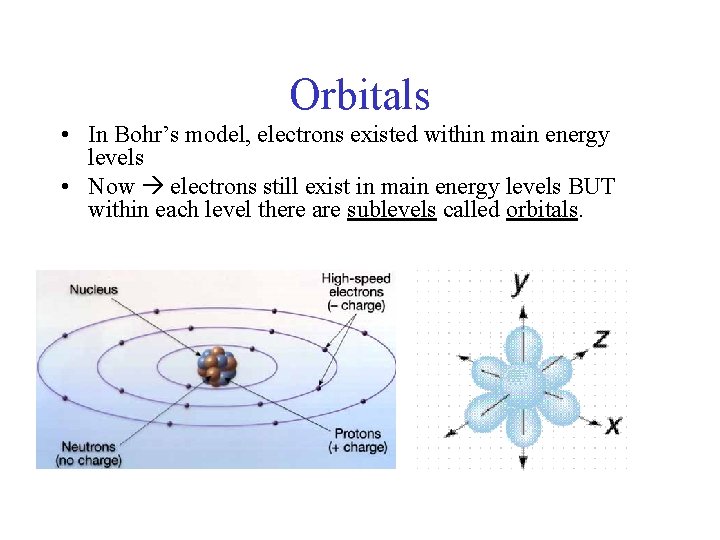
Orbitals • In Bohr’s model, electrons existed within main energy levels • Now electrons still exist in main energy levels BUT within each level there are sublevels called orbitals.

Principal Quantum Number, n • The principal quantum number represents the shell or main energy level. • Main energy levels are given values: n = 1, 2, 3, 4… • The shell or main energy level indicates the size of an orbital (or how far away the electron is from the nucleus) the higher the number, the larger the orbital (or the farther the electron). • As n increases, so does energy.
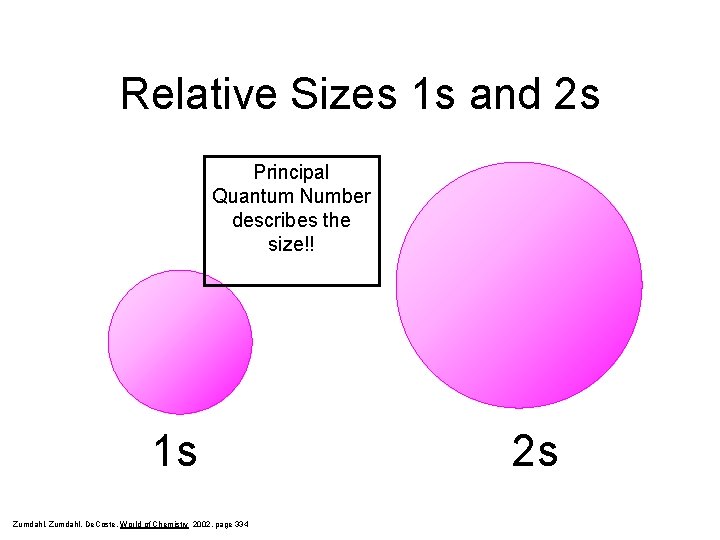
Relative Sizes 1 s and 2 s Principal Quantum Number describes the size!! 1 s Zumdahl, De. Coste, World of Chemistry 2002, page 334 2 s

Secondary Quantum Number, l • The secondary quantum number divides the shells or main energy levels up into smaller groups of subshells called orbitals it also describes the shape of orbital.
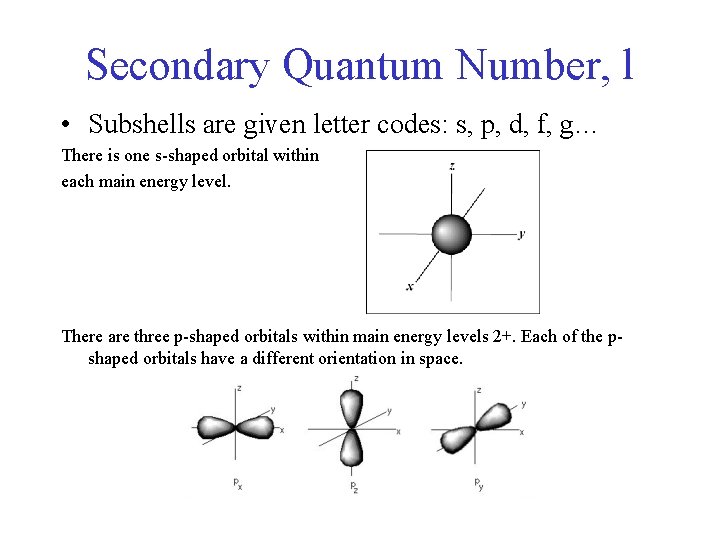
Secondary Quantum Number, l • Subshells are given letter codes: s, p, d, f, g… There is one s-shaped orbital within each main energy level. There are three p-shaped orbitals within main energy levels 2+. Each of the pshaped orbitals have a different orientation in space.
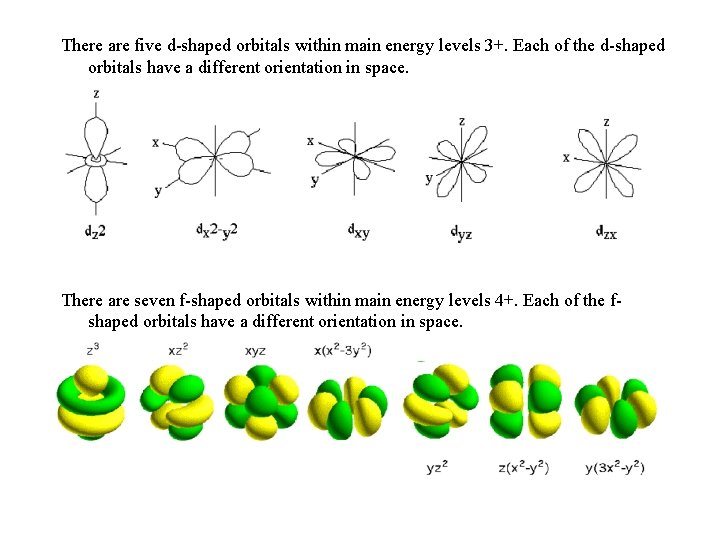
There are five d-shaped orbitals within main energy levels 3+. Each of the d-shaped orbitals have a different orientation in space. There are seven f-shaped orbitals within main energy levels 4+. Each of the fshaped orbitals have a different orientation in space.

Secondary Quantum Number, l • Within a given shell, the subshells (orbitals) are in order of increasing energy: • 4 s < 4 p < 4 d < 4 f (increasing energy)
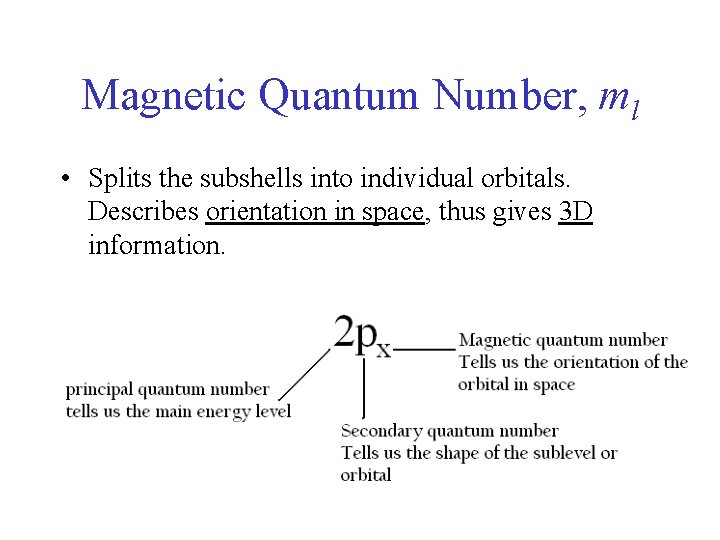
Magnetic Quantum Number, ml • Splits the subshells into individual orbitals. Describes orientation in space, thus gives 3 D information.
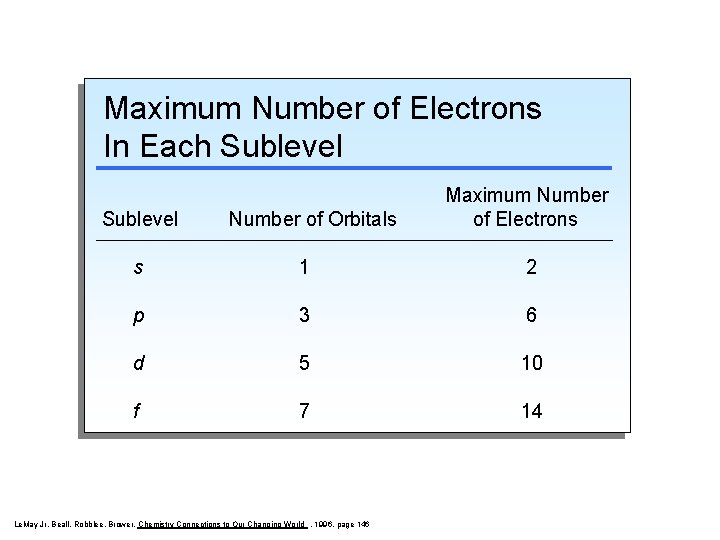
Maximum Number of Electrons In Each Sublevel Number of Orbitals Maximum Number of Electrons s 1 2 p 3 6 d 5 10 f 7 14 Le. May Jr, Beall, Robblee, Brower, Chemistry Connections to Our Changing World , 1996, page 146
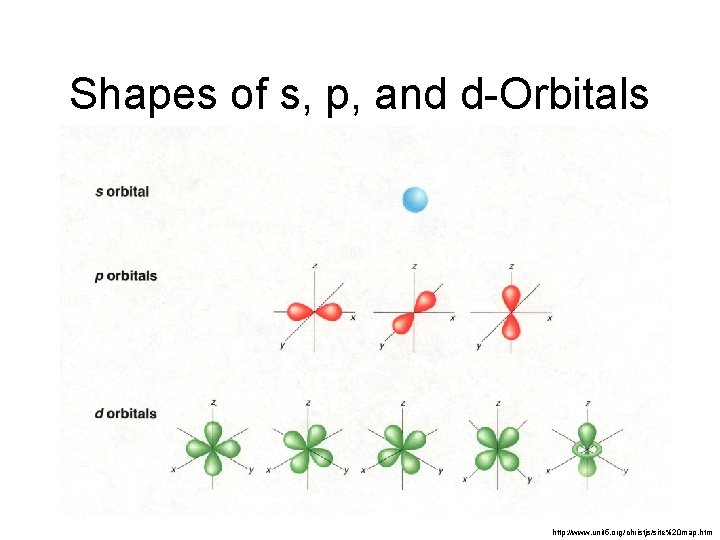
Shapes of s, p, and d-Orbitals http: //www. unit 5. org/christjs/site%20 map. htm
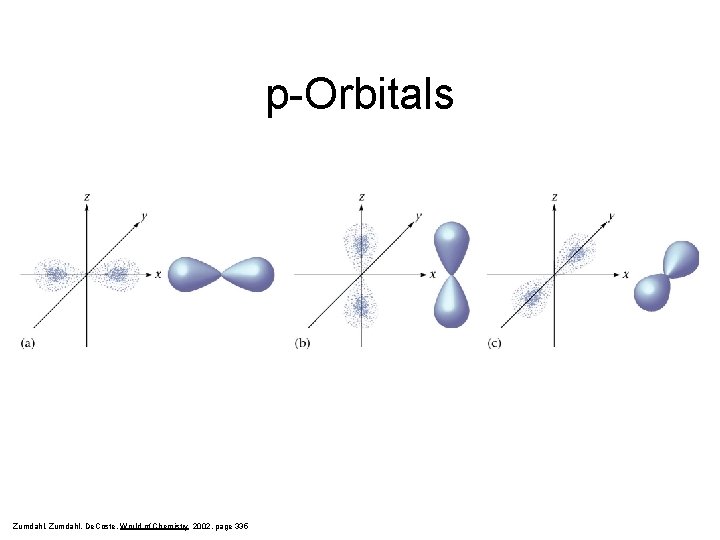
p-Orbitals Zumdahl, De. Coste, World of Chemistry 2002, page 335

Spin Magnetic Number, ms • Indicates the orientation of the two electrons in each orbital. • Values are +1/2 or – 1/2
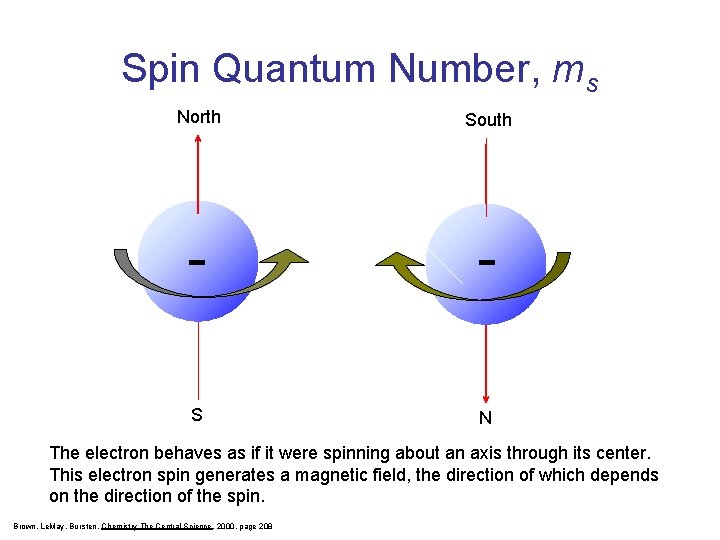
Spin Quantum Number, ms North South - - S N The electron behaves as if it were spinning about an axis through its center. This electron spin generates a magnetic field, the direction of which depends on the direction of the spin. Brown, Le. May, Bursten, Chemistry The Central Science, 2000, page 208
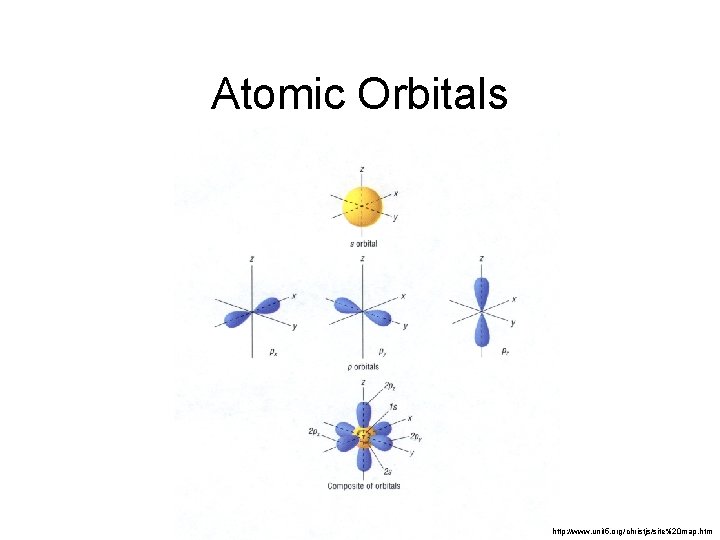
Atomic Orbitals http: //www. unit 5. org/christjs/site%20 map. htm
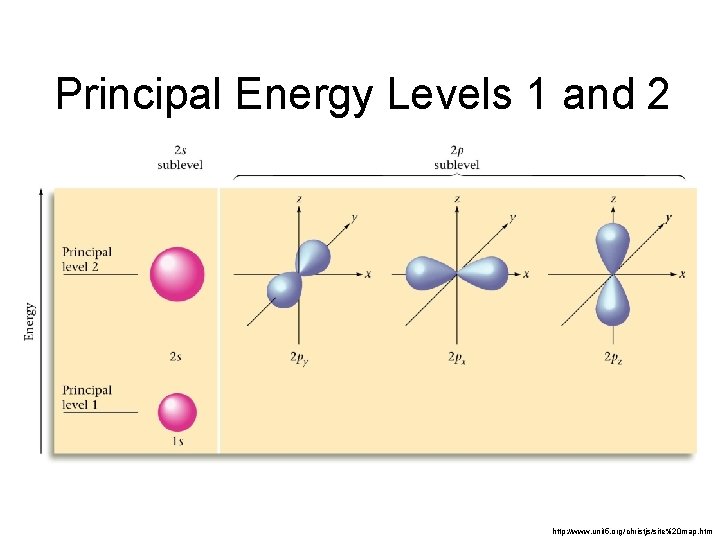
Principal Energy Levels 1 and 2 http: //www. unit 5. org/christjs/site%20 map. htm
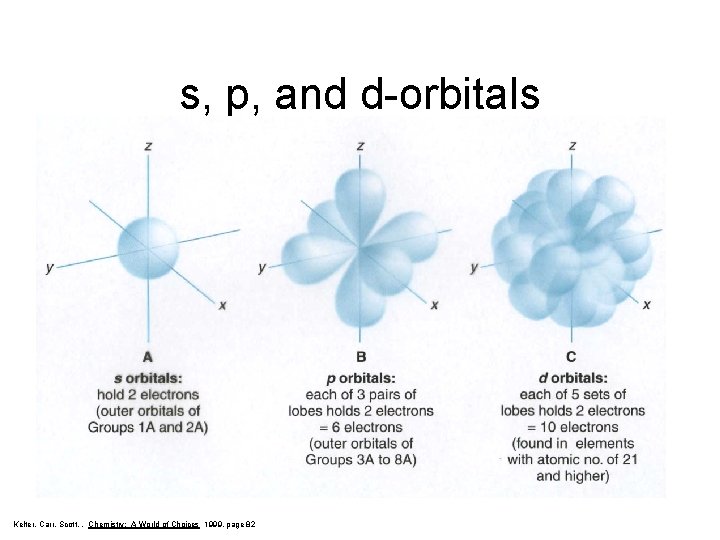
s, p, and d-orbitals Kelter, Carr, Scott, , Chemistry: A World of Choices 1999, page 82
Electron Configurations

How do we fill orbitals? • Atom is most stable when electrons have the lowest possible energy. • That is when they occupy the lowest possible energy orbitals available.

Filling Rules for Electron Orbitals Aufbau Principle: Electrons are added one at a time to the lowest energy orbitals available until all the electrons of the atom have been accounted for. Pauli Exclusion Principle: An orbital can hold a maximum of two electrons. To occupy the same orbital, two electrons must spin in opposite directions. NO TWO ELECTRONS CAN HAVE THE SAME FOUR QUANTUM NUMBERS!! Hund’s Rule: Within a given set of orbitals, one electron will go in each orbital before pairing-up begins.
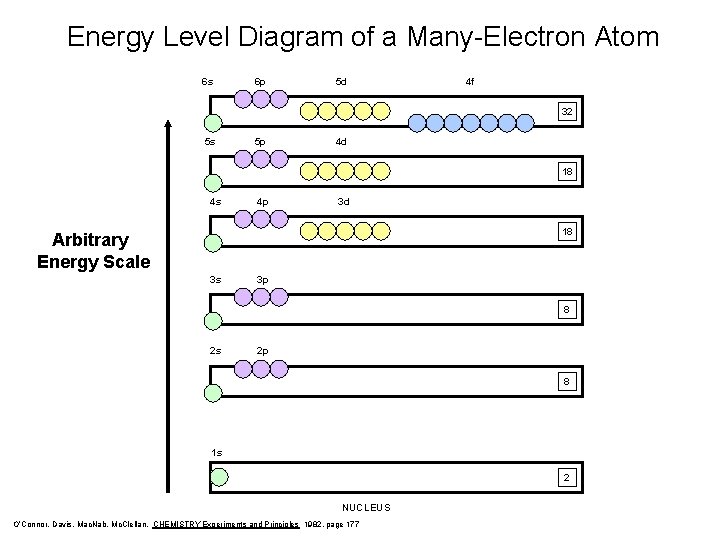
Energy Level Diagram of a Many-Electron Atom 6 s 6 p 5 d 4 f 32 5 s 5 p 4 d 18 4 s 4 p 3 d 18 Arbitrary Energy Scale 3 s 3 p 8 2 s 2 p 8 1 s 2 NUCLEUS O’Connor, Davis, Mac. Nab, Mc. Clellan, CHEMISTRY Experiments and Principles 1982, page 177
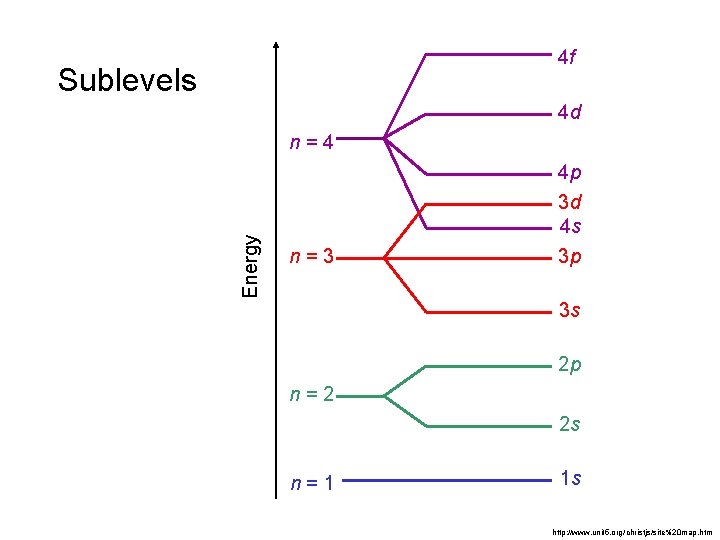
4 f Sublevels 4 d Energy n=4 n=3 4 p 3 d 4 s 3 p 3 s 2 p n=2 2 s n=1 1 s http: //www. unit 5. org/christjs/site%20 map. htm
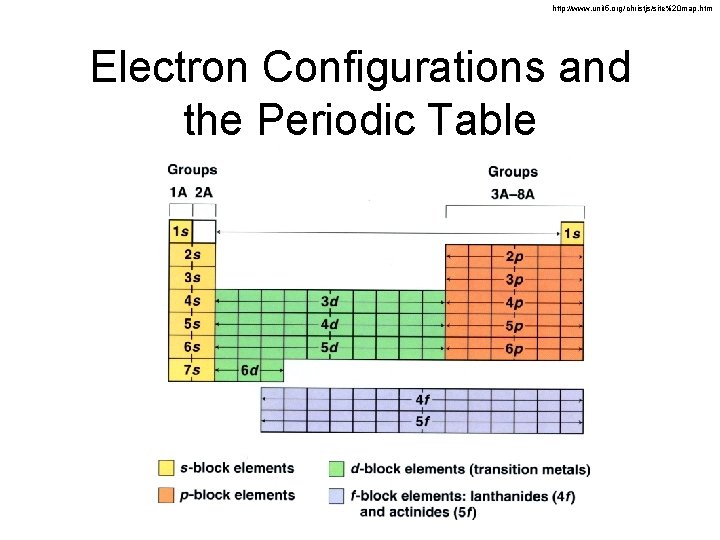
http: //www. unit 5. org/christjs/site%20 map. htm Electron Configurations and the Periodic Table
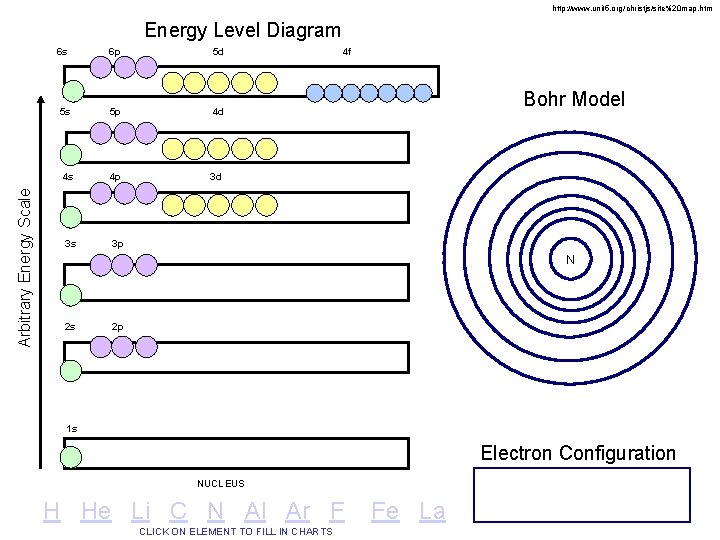
http: //www. unit 5. org/christjs/site%20 map. htm Arbitrary Energy Scale Energy Level Diagram 6 s 6 p 5 d 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p 4 f Bohr Model N 2 s 2 p 1 s Electron Configuration NUCLEUS H He Li C N Al Ar F CLICK ON ELEMENT TO FILL IN CHARTS Fe La
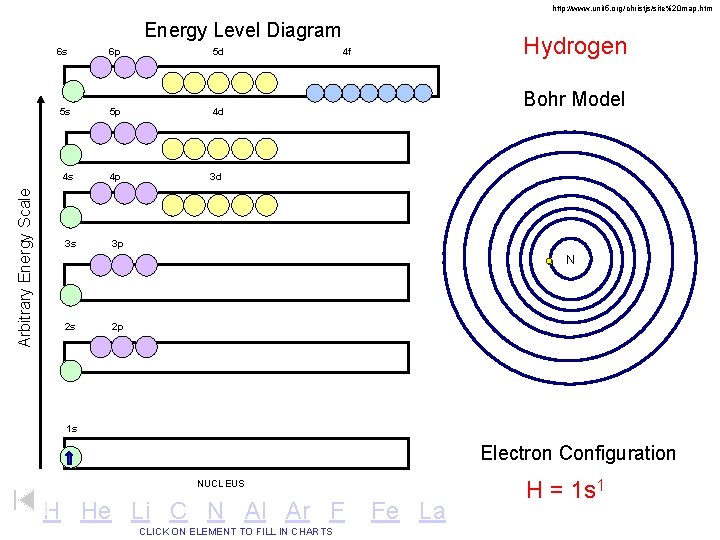
http: //www. unit 5. org/christjs/site%20 map. htm Arbitrary Energy Scale Energy Level Diagram 6 s 6 p 5 d 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p Hydrogen 4 f Bohr Model N 2 s 2 p 1 s Electron Configuration NUCLEUS H He Li C N Al Ar F CLICK ON ELEMENT TO FILL IN CHARTS Fe La H = 1 s 1
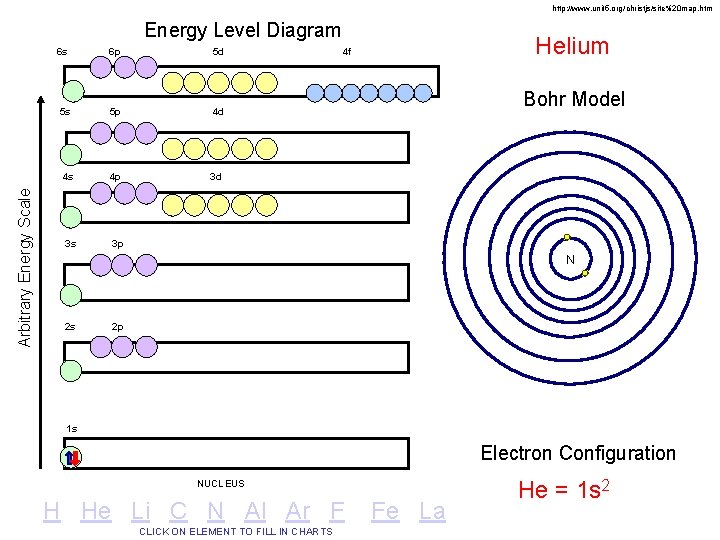
http: //www. unit 5. org/christjs/site%20 map. htm Arbitrary Energy Scale Energy Level Diagram 6 s 6 p 5 d 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p Helium 4 f Bohr Model N 2 s 2 p 1 s Electron Configuration NUCLEUS H He Li C N Al Ar F CLICK ON ELEMENT TO FILL IN CHARTS Fe La He = 1 s 2
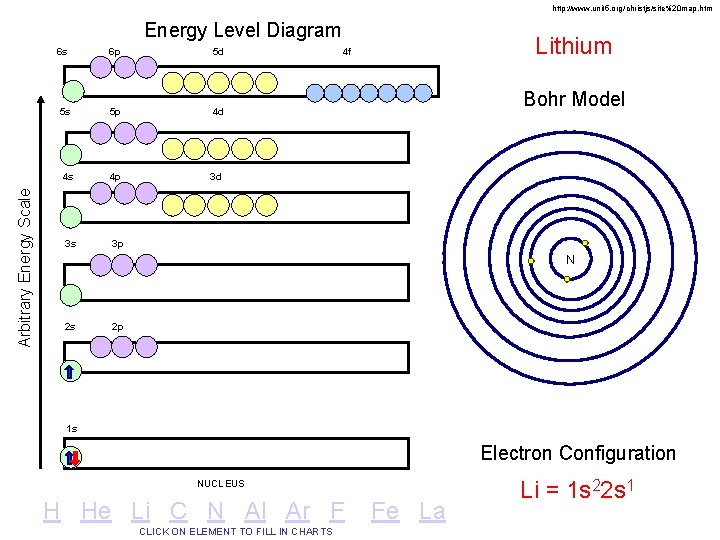
http: //www. unit 5. org/christjs/site%20 map. htm Arbitrary Energy Scale Energy Level Diagram 6 s 6 p 5 d 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p Lithium 4 f Bohr Model N 2 s 2 p 1 s Electron Configuration NUCLEUS H He Li C N Al Ar F CLICK ON ELEMENT TO FILL IN CHARTS Fe La Li = 1 s 22 s 1
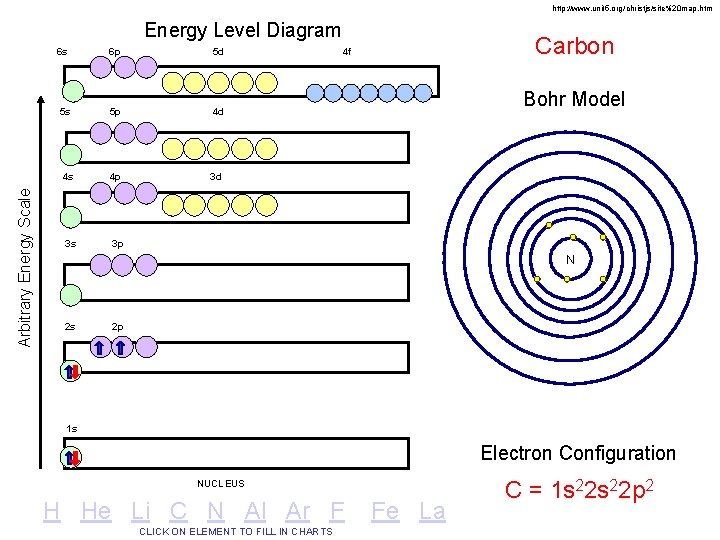
http: //www. unit 5. org/christjs/site%20 map. htm Arbitrary Energy Scale Energy Level Diagram 6 s 6 p 5 d 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p Carbon 4 f Bohr Model N 2 s 2 p 1 s Electron Configuration NUCLEUS H He Li C N Al Ar F CLICK ON ELEMENT TO FILL IN CHARTS Fe La C = 1 s 22 p 2
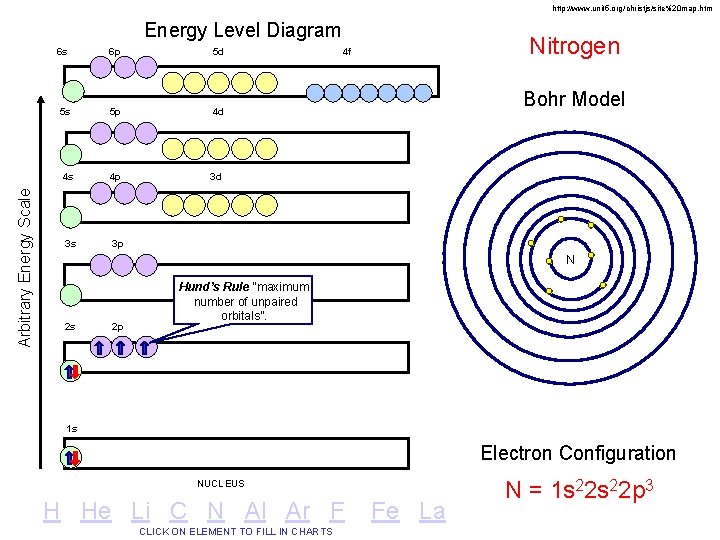
http: //www. unit 5. org/christjs/site%20 map. htm Arbitrary Energy Scale Energy Level Diagram 6 s 6 p 5 d 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p Nitrogen 4 f Bohr Model N 2 s 2 p Hund’s Rule “maximum number of unpaired orbitals”. 1 s Electron Configuration NUCLEUS H He Li C N Al Ar F CLICK ON ELEMENT TO FILL IN CHARTS Fe La N = 1 s 22 p 3
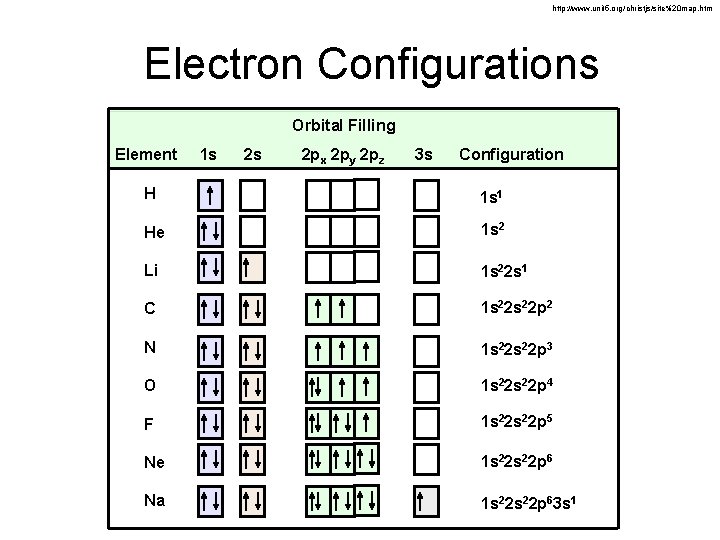
http: //www. unit 5. org/christjs/site%20 map. htm Electron Configurations Orbital Filling Element 1 s 2 s 2 px 2 py 2 pz 3 s Electron Configuration H 1 s 1 He 1 s 2 Li 1 s 22 s 1 C 1 s 22 p 2 N 1 s 22 p 3 O 1 s 22 p 4 F 1 s 22 p 5 Ne 1 s 22 p 6 Na 1 s 22 p 63 s 1
- Slides: 31