Electron Configuration of Atoms in their Ground State





- Slides: 19

Electron Configuration of Atoms in their Ground State • The electron configuration is a listing of the subshells in order of filling with the number of electrons in that subshell written as a superscript. Kr = 36 electrons = 1 s 22 p 63 s 23 p 64 s 23 d 104 p 6 • A short-hand way of writing an electron configuration is to use the symbol of the previous noble gas in [] to represent all the inner electrons, then just write the last set. Rb = 37 electrons = 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 1 = [Kr]5 s 1 Tro's "Introductory Chemistry", Chapter 9 1

Practice—Write the Full Ground State Orbital Diagram and Electron Configuration of Potassium. Tro's "Introductory Chemistry", Chapter 9 2

Practice—Write the Full Ground State Orbital Diagram and Electron Configuration of F−. Tro's "Introductory Chemistry", Chapter 9 3

Valence Electrons • The electrons in all the subshells with the highest principal energy shells are called the valence electrons. • Electrons in lower energy shells are called core electrons. • Chemists have observed that one of the most important factors in the way an atom behaves, both chemically and physically, is the number of valence electrons. Tro's "Introductory Chemistry", Chapter 9 4

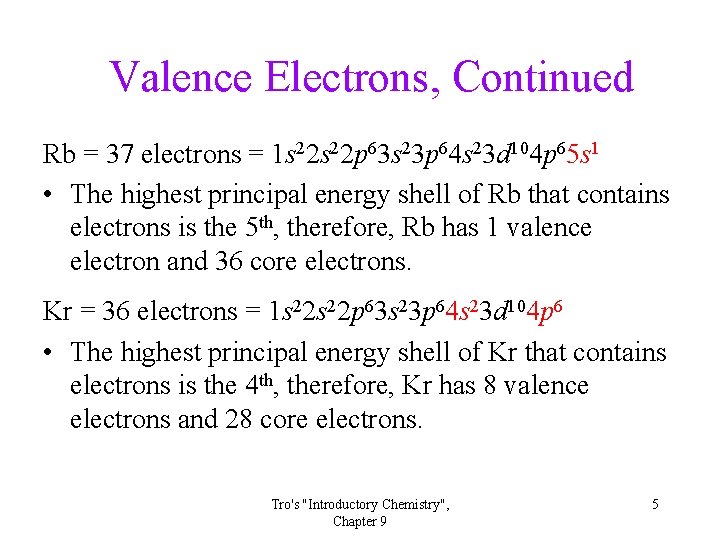
Valence Electrons, Continued Rb = 37 electrons = 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 1 • The highest principal energy shell of Rb that contains electrons is the 5 th, therefore, Rb has 1 valence electron and 36 core electrons. Kr = 36 electrons = 1 s 22 p 63 s 23 p 64 s 23 d 104 p 6 • The highest principal energy shell of Kr that contains electrons is the 4 th, therefore, Kr has 8 valence electrons and 28 core electrons. Tro's "Introductory Chemistry", Chapter 9 5

Practice—Determine the Number and Types of Valence Electrons in an Arsenic, As, Atom. Tro's "Introductory Chemistry", Chapter 9 6

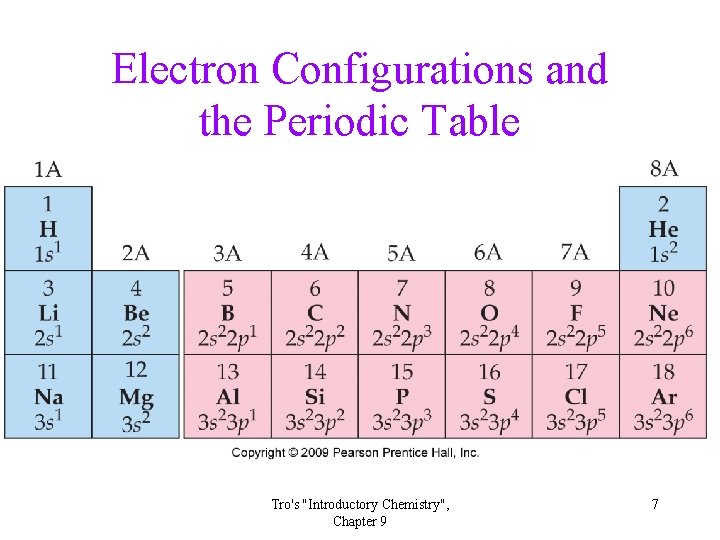
Electron Configurations and the Periodic Table Tro's "Introductory Chemistry", Chapter 9 7

Electron Configurations from the Periodic Table • Elements in the same period (row) have valence electrons in the same principal energy shell. • The number of valence electrons increases by one as you progress across the period. • Elements in the same group (column) have the same number of valence electrons and the valence electrons are in the same type of subshell. Tro's "Introductory Chemistry", Chapter 9 8

Electron Configuration and the Periodic Table • Elements in the same column have similar chemical and physical properties because their valence shell electron configuration is the same. • The number of valence electrons for the main group elements is the same as the group number. Tro's "Introductory Chemistry", Chapter 9 9

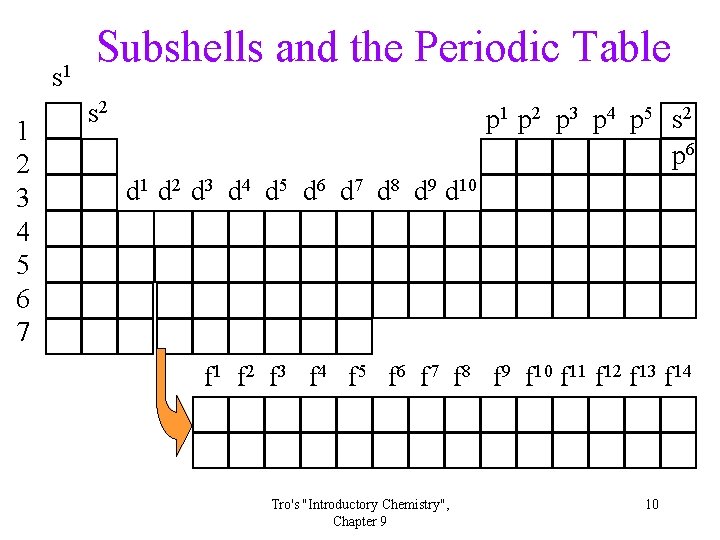
s 1 1 2 3 4 5 6 7 Subshells and the Periodic Table s 2 p 1 p 2 p 3 p 4 p 5 s 2 p 6 d 1 d 2 d 3 d 4 d 5 d 6 d 7 d 8 d 9 d 10 f 1 f 2 f 3 f 4 f 5 f 6 f 7 f 8 f 9 f 10 f 11 f 12 f 13 f 14 Tro's "Introductory Chemistry", Chapter 9 10

Electron Configuration from the Periodic Table • The inner electron configuration is the same as the noble gas of the preceding period. • To get the outer electron configuration from the preceding noble gas, loop through the next period, marking the subshells as you go, until you reach the element. ü The valence energy shell = the period number. ü The d block is always one energy shell below the period number and the f is two energy shells below. Tro's "Introductory Chemistry", Chapter 9 11

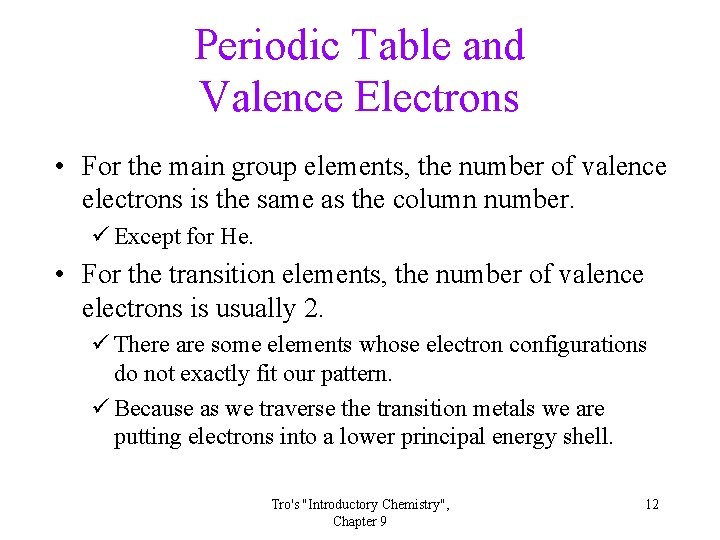
Periodic Table and Valence Electrons • For the main group elements, the number of valence electrons is the same as the column number. ü Except for He. • For the transition elements, the number of valence electrons is usually 2. ü There are some elements whose electron configurations do not exactly fit our pattern. ü Because as we traverse the transition metals we are putting electrons into a lower principal energy shell. Tro's "Introductory Chemistry", Chapter 9 12

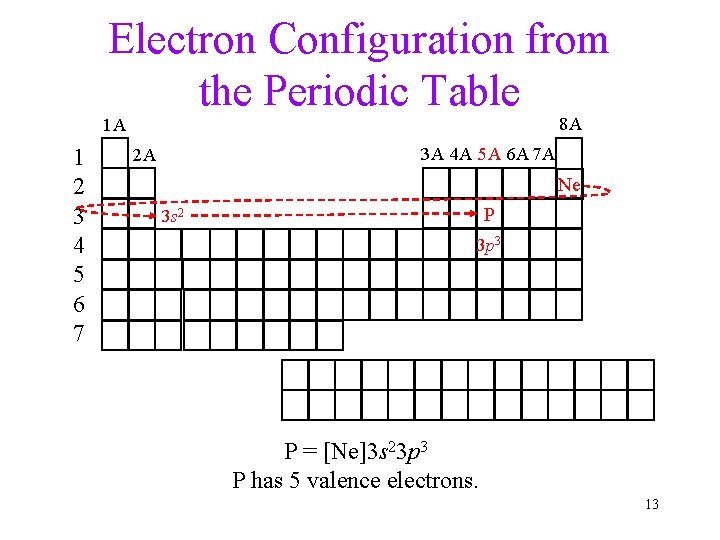
Electron Configuration from the Periodic Table 8 A 1 A 1 2 3 4 5 6 7 3 A 4 A 5 A 6 A 7 A 2 A Ne P 3 s 2 3 p 3 P = [Ne]3 s 23 p 3 P has 5 valence electrons. 13

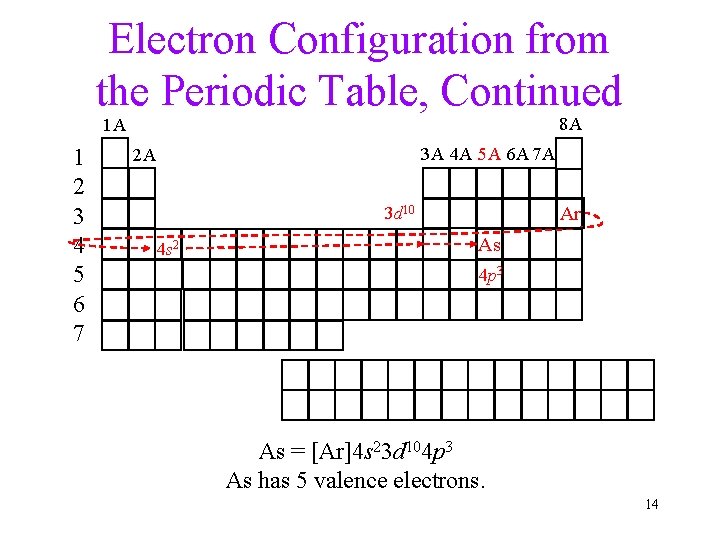
Electron Configuration from the Periodic Table, Continued 8 A 1 A 1 2 3 4 5 6 7 3 A 4 A 5 A 6 A 7 A 2 A Ar 3 d 10 4 s 2 As 4 p 3 As = [Ar]4 s 23 d 104 p 3 As has 5 valence electrons. 14

Practice—Use the Periodic Table to Write the Short Electron Configuration and Orbital Diagram for Each of the Following and Determine the Number of Valence Electrons. • Na (at. no. 11). • Te (at. no. 52). Tro's "Introductory Chemistry", Chapter 9 15

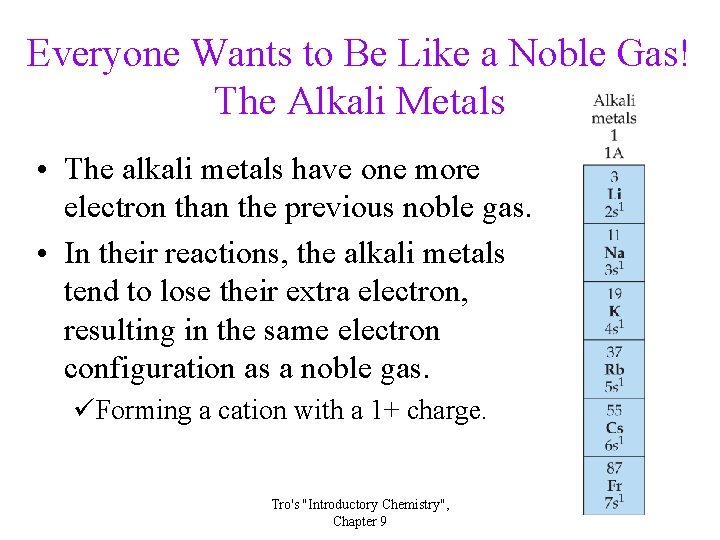
Everyone Wants to Be Like a Noble Gas! The Alkali Metals • The alkali metals have one more electron than the previous noble gas. • In their reactions, the alkali metals tend to lose their extra electron, resulting in the same electron configuration as a noble gas. üForming a cation with a 1+ charge. Tro's "Introductory Chemistry", Chapter 9

Everyone Wants to Be Like a Noble Gas! The Halogens • The electron configurations of the halogens all have one fewer electron than the next noble gas. • In their reactions with metals, the halogens tend to gain an electron and attain the electron configuration of the next noble gas. ü Forming an anion with charge 1−. • In their reactions with nonmetals, they tend to share electrons with the other nonmetal so that each attains the electron configuration of a noble gas. Tro's "Introductory Chemistry", Chapter 9 17

Everyone Wants to Be Like a Noble Gas! • As a group, the alkali metals are the most reactive metals. üThey react with many things and do so rapidly. • The halogens are the most reactive group of nonmetals. • One reason for their high reactivity is the fact that they are only one electron away from having a very stable electron configuration. üThe same as a noble gas. Tro's "Introductory Chemistry", Chapter 9 18

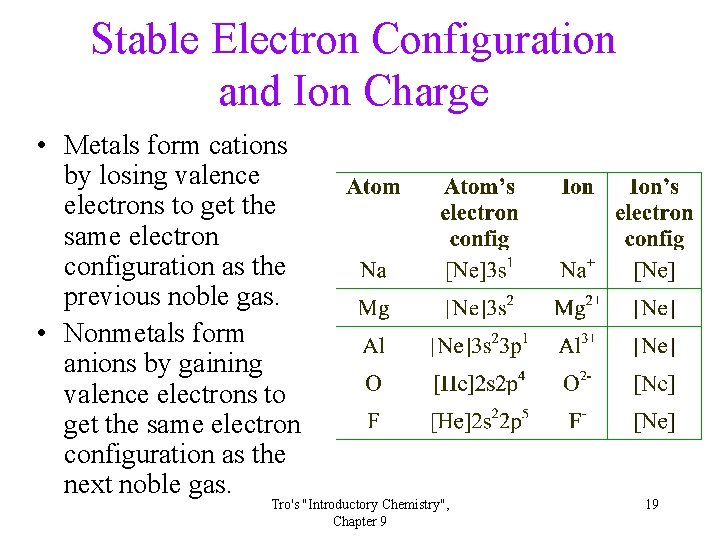
Stable Electron Configuration and Ion Charge • Metals form cations by losing valence electrons to get the same electron configuration as the previous noble gas. • Nonmetals form anions by gaining valence electrons to get the same electron configuration as the next noble gas. Tro's "Introductory Chemistry", Chapter 9 19