Electron Configuration FillingOrder of Electrons in an Atom







































![Shorthand Configuration Element symbol Electron configuration Ca [Ar] 4 s 2 V [Ar] 4 Shorthand Configuration Element symbol Electron configuration Ca [Ar] 4 s 2 V [Ar] 4](https://slidetodoc.com/presentation_image_h/ee5c2adeea6e19453aab4110c84c4a3b/image-41.jpg)
- Slides: 41

Electron Configuration Filling-Order of Electrons in an Atom

Electron capacities Copyright © 2006 Pearson Benjamin Cummings. All rights reserved.

Periodic Patterns s 1 2 3 4 5 6 7 p 1 s 2 s f 2 p 3 s d (n-1) 4 s 3 d 4 p 5 s 4 d 5 p 6 s 5 d 6 p 7 s 6 d 7 p 6 (n-2) 7 3 p 4 f 5 f 1 s

Electron Filling in Periodic Table s s 1 H p H He 1 s 1 1 s 2 1 s 1 2 3 4 5 6 7 Li Be B C N O F Ne 2 s 1 2 s 2 2 p 1 2 p 2 2 p 3 2 p 4 2 p 5 2 p 6 Al Si P S Cl Ar 3 p 1 3 p 2 3 p 3 3 p 4 3 p 5 3 p 6 Na Mg d 3 s 1 3 s 2 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 4 s 1 4 s 2 3 d 1 3 d 2 3 d 3 3 d 5 3 d 10 4 p 1 4 p 2 4 p 5 4 p 6 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe 5 s 1 4 d 2 4 d 4 4 d 5 4 d 6 4 d 7 4 d 8 4 d 10 4 p 1 5 p 2 5 p 3 5 p 4 5 p 5 5 p 6 Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn 5 d 2 5 d 3 5 d 4 5 d 5 5 d 7 5 d 9 6 p 1 6 p 2 6 p 3 6 p 4 5 s 2 Cs Ba 6 s 1 6 s 2 Fr Ra 7 s 1 7 s 2 * W 5 d 6 3 d 7 3 d 8 3 d 10 4 d 10 5 d 10 4 p 3 4 p 4 6 p 5 6 p 6 6 d 3 6 d 4 6 d 5 6 d 6 6 d 7 f La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu 5 d 1 W 3 d 6 Rf Db Sg Bh Hs Mt 6 d 2 * 3 d 5 4 f 2 4 f 3 4 f 4 Ac Th Pa U 6 d 1 5 f 3 6 d 2 5 f 2 4 f 5 4 f 6 4 f 7 4 f 9 4 f 10 Np Pu Am Cm Bk Cf 5 f 4 5 f 6 5 f 7 5 f 8 5 f 10 4 f 11 4 f 12 4 f 13 4 f 14 4 f 114 Es Fm Md No Lr 5 f 11 5 f 14 5 f 13 5 f 14

4 f Sublevels 4 d Energy n=4 n=3 4 p 3 d 4 s 3 p 3 s 2 p n=2 2 s n=1 1 s

4 f Sublevels 4 d s p s d p s n=4 f d p Energy s n=3 4 p 3 d 4 s 3 p 3 s 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 24 d 10… 2 p n=2 2 s n=1 1 s

Filling Rules for Electron Orbitals Aufbau Principle: Electrons are added one at a time to the lowest energy orbitals available until all the electrons of the atom have been accounted for. Pauli Exclusion Principle: An orbital can hold a maximum of two electrons. To occupy the same orbital, two electrons must spin in opposite directions. Hund’s Rule: Electrons occupy equal-energy orbitals so that a maximum number of unpaired electrons results. *Aufbau is German for “building up”

Copyright © 2007 Pearson Benjamin Cummings. All rights reserved.

Energy Level Diagram of a Many-Electron Atom 6 s 6 p 5 d 4 f 32 5 s 5 p 4 d 18 4 s 4 p 3 d 18 Arbitrary Energy Scale 3 s 3 p 8 2 s 2 p 8 1 s 2 NUCLEUS O’Connor, Davis, Mac. Nab, Mc. Clellan, CHEMISTRY Experiments and Principles 1982, page 177

Arbitrary Energy Scale Energy Level Diagram 6 s 6 p 5 d 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p 4 f Bohr Model N 2 s 2 p 1 s Electron Configuration NUCLEUS H He Li C N Al Ar F CLICK ON ELEMENT TO FILL IN CHARTS Fe La

Arbitrary Energy Scale Energy Level Diagram 6 s 6 p 5 d 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p Hydrogen 4 f Bohr Model N 2 s 2 p 1 s Electron Configuration NUCLEUS H He Li C N Al Ar F CLICK ON ELEMENT TO FILL IN CHARTS Fe La H = 1 s 1

Arbitrary Energy Scale Energy Level Diagram 6 s 6 p 5 d 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p Helium 4 f Bohr Model N 2 s 2 p 1 s Electron Configuration NUCLEUS H He Li C N Al Ar F CLICK ON ELEMENT TO FILL IN CHARTS Fe La He = 1 s 2

Arbitrary Energy Scale Energy Level Diagram 6 s 6 p 5 d 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p Lithium 4 f Bohr Model N 2 s 2 p 1 s Electron Configuration NUCLEUS H He Li C N Al Ar F CLICK ON ELEMENT TO FILL IN CHARTS Fe La Li = 1 s 22 s 1

Arbitrary Energy Scale Energy Level Diagram 6 s 6 p 5 d 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p Carbon 4 f Bohr Model N 2 s 2 p 1 s Electron Configuration NUCLEUS H He Li C N Al Ar F CLICK ON ELEMENT TO FILL IN CHARTS Fe La C = 1 s 22 p 2

Arbitrary Energy Scale Energy Level Diagram 6 s 6 p 5 d 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p Nitrogen 4 f Bohr Model N 2 s 2 p Hund’s Rule “maximum number of unpaired orbitals”. 1 s Electron Configuration NUCLEUS H He Li C N Al Ar F CLICK ON ELEMENT TO FILL IN CHARTS Fe La N = 1 s 22 p 3

Arbitrary Energy Scale Energy Level Diagram 6 s 6 p 5 d 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p Fluorine 4 f Bohr Model N 2 s 2 p 1 s Electron Configuration NUCLEUS H He Li C N Al Ar F CLICK ON ELEMENT TO FILL IN CHARTS Fe La F = 1 s 22 p 5

Arbitrary Energy Scale Energy Level Diagram 6 s 6 p 5 d 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p Aluminum 4 f Bohr Model N 2 s 2 p 1 s Electron Configuration NUCLEUS H He Li C N Al Ar F CLICK ON ELEMENT TO FILL IN CHARTS Fe La Al = 1 s 22 p 63 s 23 p 1

Arbitrary Energy Scale Energy Level Diagram 6 s 6 p 5 d 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p Argon 4 f Bohr Model N 2 s 2 p 1 s Electron Configuration NUCLEUS H He Li C N Al Ar F CLICK ON ELEMENT TO FILL IN CHARTS Fe La Ar = 1 s 22 p 63 s 23 p 6

Arbitrary Energy Scale Energy Level Diagram 6 s 6 p 5 d 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p Iron 4 f Bohr Model N 2 s 2 p 1 s Electron Configuration NUCLEUS H He Li C N Al Ar F CLICK ON ELEMENT TO FILL IN CHARTS Fe = 1 s 22 p 63 s 23 p 64 s 23 d 6 Fe La

Arbitrary Energy Scale Energy Level Diagram 6 s 6 p 5 d 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p Lanthanum 4 f Bohr Model N 2 s 2 p 1 s Electron Configuration NUCLEUS H He Li C N Al Ar F CLICK ON ELEMENT TO FILL IN CHARTS Fe La La = 1 s 22 p 63 s 23 p 64 s 23 d 6 4 s 23 d 104 p 65 s 24 d 105 p 66 s 25 d 1

Energy Level Diagram 6 s 6 p 5 d 4 f Bohr Model Arbitrary Energy Scale 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p 2 s 2 p 1 s Electron Configuration NUCLEUS H He Li C N O F

Energy Level Diagram 6 s 6 p 5 d 4 f Bohr Model Arbitrary Energy Scale 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p 2 s 2 p 1 s Electron Configuration NUCLEUS H He Li C N O F

Energy Level Diagram 6 s 6 p 5 d 4 f Bohr Model Arbitrary Energy Scale 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p 2 s 2 p 1 s Electron Configuration NUCLEUS H He Li C N O F

Arbitrary Energy Scale Energy Level Diagram 6 s 6 p 5 d 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p Hydrogen 4 f Bohr Model N 2 s 2 p 1 s Electron Configuration NUCLEUS H He Li C N Al Ar F CLICK ON ELEMENT TO FILL IN CHARTS Fe La H = 1 s 1

Arbitrary Energy Scale Energy Level Diagram 6 s 6 p 5 d 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p Helium 4 f Bohr Model N 2 s 2 p 1 s Electron Configuration NUCLEUS H He Li C N Al Ar F CLICK ON ELEMENT TO FILL IN CHARTS Fe La He = 1 s 2

Arbitrary Energy Scale Energy Level Diagram 6 s 6 p 5 d 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p Lithium 4 f Bohr Model N 2 s 2 p 1 s Electron Configuration NUCLEUS H He Li C N Al Ar F CLICK ON ELEMENT TO FILL IN CHARTS Fe La Li = 1 s 22 s 1

Arbitrary Energy Scale Energy Level Diagram 6 s 6 p 5 d 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p Carbon 4 f Bohr Model N 2 s 2 p 1 s Electron Configuration NUCLEUS H He Li C N Al Ar F CLICK ON ELEMENT TO FILL IN CHARTS Fe La C = 1 s 22 p 2

Arbitrary Energy Scale Energy Level Diagram 6 s 6 p 5 d 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p Nitrogen 4 f Bohr Model N 2 s 2 p Hund’s Rule “maximum number of unpaired orbitals”. 1 s Electron Configuration NUCLEUS H He Li C N Al Ar F CLICK ON ELEMENT TO FILL IN CHARTS Fe La N = 1 s 22 p 3

Arbitrary Energy Scale Energy Level Diagram 6 s 6 p 5 d 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p Fluorine 4 f Bohr Model N 2 s 2 p 1 s Electron Configuration NUCLEUS H He Li C N Al Ar F CLICK ON ELEMENT TO FILL IN CHARTS Fe La F = 1 s 22 p 5

Arbitrary Energy Scale Energy Level Diagram 6 s 6 p 5 d 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p Aluminum 4 f Bohr Model N 2 s 2 p 1 s Electron Configuration NUCLEUS H He Li C N Al Ar F CLICK ON ELEMENT TO FILL IN CHARTS Fe La Al = 1 s 22 p 63 s 23 p 1

Arbitrary Energy Scale Energy Level Diagram 6 s 6 p 5 d 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p Argon 4 f Bohr Model N 2 s 2 p 1 s Electron Configuration NUCLEUS H He Li C N Al Ar F CLICK ON ELEMENT TO FILL IN CHARTS Fe La Ar = 1 s 22 p 63 s 23 p 6

Arbitrary Energy Scale Energy Level Diagram 6 s 6 p 5 d 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p 2 s 2 p 4 f Iron Bohr Model N 1 s Electron Configuration NUCLEUS H He Li C N Al Ar F CLICK ON ELEMENT TO FILL IN CHARTS Fe = 1 s 22 p 63 s 23 p 64 s 23 d 6 Fe La

Energy Level Diagram 6 s 6 p 5 d Lanthanum 4 f Bohr Model Arbitrary Energy Scale 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p 2 s 2 p N 1 s Electron Configuration NUCLEUS H He Li C N Al O F CLICK ON ELEMENT TO FILL IN CHARTS Fe La La = 1 s 22 p 63 s 23 p 64 s 23 d 6 4 s 23 d 104 p 65 s 24 d 105 p 66 s 25 d 1

Energy Level Diagram 6 s 6 p 5 d 4 f Bohr Model Arbitrary Energy Scale 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p 2 s 2 p 1 s Electron Configuration NUCLEUS H He Li C N O F

Energy Level Diagram 6 s 6 p 5 d 4 f Bohr Model Arbitrary Energy Scale 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p 2 s 2 p 1 s Electron Configuration NUCLEUS H He Li C N O CLICK ON ELEMENT TO FILL IN CHARTS F H = 1 s 1

Shorthand Electron Configuration

H = 1 s 1 1 s 2 s 2 px 2 py 2 pz 3 s 3 px 3 py 3 pz 1 s 2 s 2 px 2 py 2 pz 3 s 3 px 3 py 3 pz He = 1 s 2 Li = 1 s 2 2 s 1 Be = 1 s 1 2 s 2 C = 1 s 2 2 p 2 S = 1 s 2 2 p 6 3 s 2 3 p 4

H = 1 s 1 e- 1 s 2 s 2 px 2 py 2 pz 3 s 3 px 3 py 3 pz +1 He = 1 s 2 ee- +2 Coulombic attraction holds valence electrons to atom. Be = 1 s 2 2 s 2 1 s 2 s 2 px 2 py 2 pz 3 s 3 px 3 py 3 pz eee- Coulombic attraction holds valence electrons to atom. +4 e- Valence electrons are shielded by the kernel electrons. Therefore the valence electrons are not held as tightly in Be than in He. This is why a 2 s orbital (electron cloud) is larger than a 1 s orbital.

26 1 s 2 px 2 py 2 pz 2 s 3 s Fe 26 electrons. Iron has ___ Fe = 1 s 2 2 s 22 p 63 s 23 p 64 s 23 d 6 3 px 3 py 3 pz 6 s 6 p 4 s 5 d 3 d 3 d 55. 85 3 d 4 f 32 5 s e- eee- e- e- +26 e- e- ee- e- e- 4 s 4 p 3 d e- ee- e- e- 4 d 18 e- e- 5 p 18 Arbitrary Energy Scale 3 s 3 p 8 ee- 2 s 2 p 8 Bohr model of Iron 1 s 2 NUCLEUS 3 d 3 d

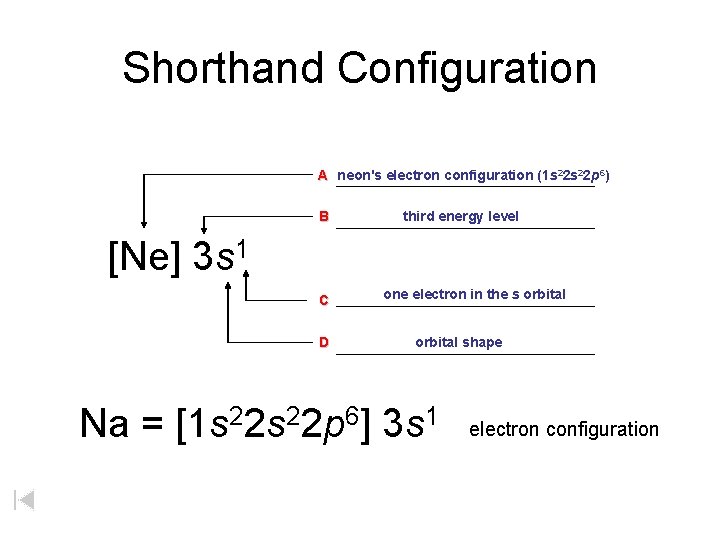
Shorthand Configuration A neon's electron configuration (1 s 22 p 6) B third energy level [Ne] 3 s 1 C D one electron in the s orbital shape Na = [1 s 22 p 6] 3 s 1 electron configuration
![Shorthand Configuration Element symbol Electron configuration Ca Ar 4 s 2 V Ar 4 Shorthand Configuration Element symbol Electron configuration Ca [Ar] 4 s 2 V [Ar] 4](https://slidetodoc.com/presentation_image_h/ee5c2adeea6e19453aab4110c84c4a3b/image-41.jpg)
Shorthand Configuration Element symbol Electron configuration Ca [Ar] 4 s 2 V [Ar] 4 s 2 3 d 3 F [He] 2 s 2 2 p 5 Ag [Kr] 5 s 2 4 d 9 I [Kr] 5 s 2 4 d 10 5 p 5 Xe [Kr] 5 s 2 4 d 10 5 p 6 Fe Sg 22 p 64 s [He] 2 s[Ar] 3 s 223 d 3 p 664 s 23 d 6 [Rn] 7 s 2 5 f 14 6 d 4