Electron Configuration Electron Configuration Like the electron address


- Slides: 14

Electron Configuration

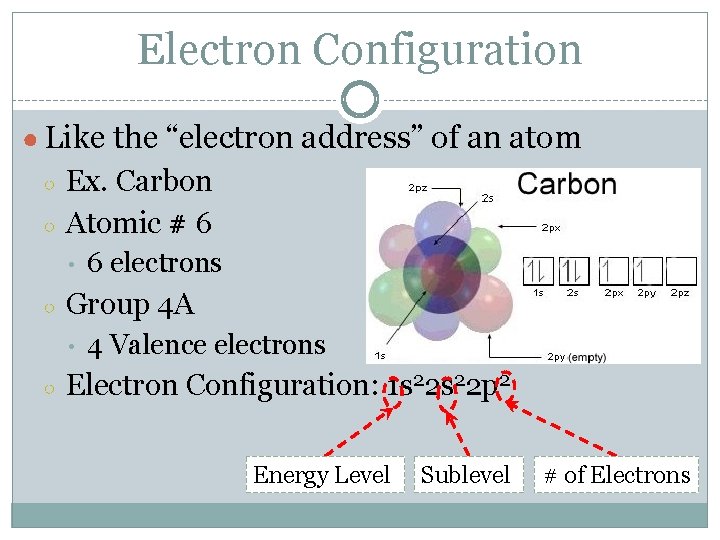
Electron Configuration ● Like the “electron address” of an atom ○ ○ Ex. Carbon Atomic # 6 • ○ Group 4 A • ○ 6 electrons 4 Valence electrons Electron Configuration: 1 s 22 p 2 Energy Level Sublevel # of Electrons

HOW TO DETERMINE ELECTRON CONFIGURATIONS ■ How are electrons? arranged around the nuclei of atoms ■ 3 RULES: ■ ■ ■ Aufbau Principle Hund’s Rule Pauli Exclusion Principle

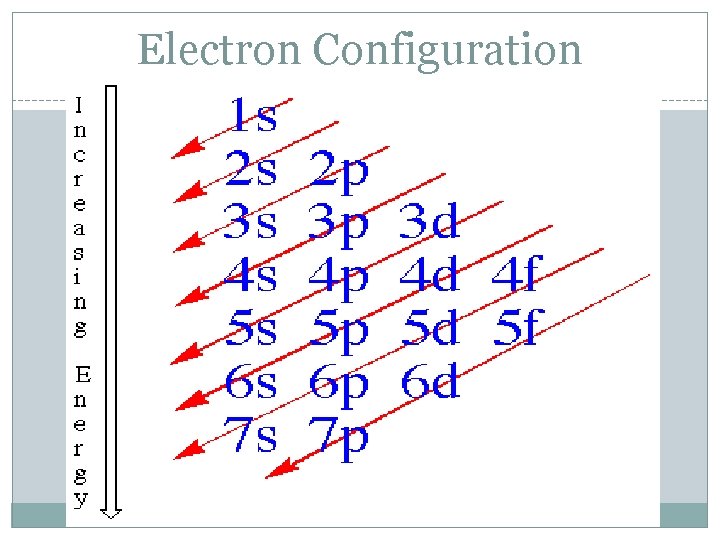
Electron Configuration ● Electrons fill orbitals following specific rules: ○ ○ Aufbau Principle: electrons always fill levels/orbitals with the lowest energy first 1 s – 2 p – 3 s – 3 p – 4 s – 3 d – 4 p … • Think of a hotel: you put guests on the first floor first, before moving up to the second floor

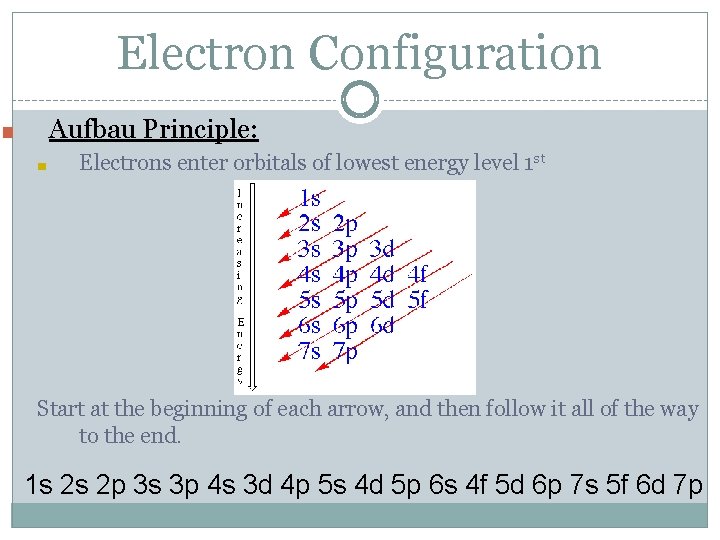
Electron Configuration Aufbau Principle: ■ ■ Electrons enter orbitals of lowest energy level 1 st Start at the beginning of each arrow, and then follow it all of the way to the end. 1 s 2 s 2 p 3 s 3 p 4 s 3 d 4 p 5 s 4 d 5 p 6 s 4 f 5 d 6 p 7 s 5 f 6 d 7 p

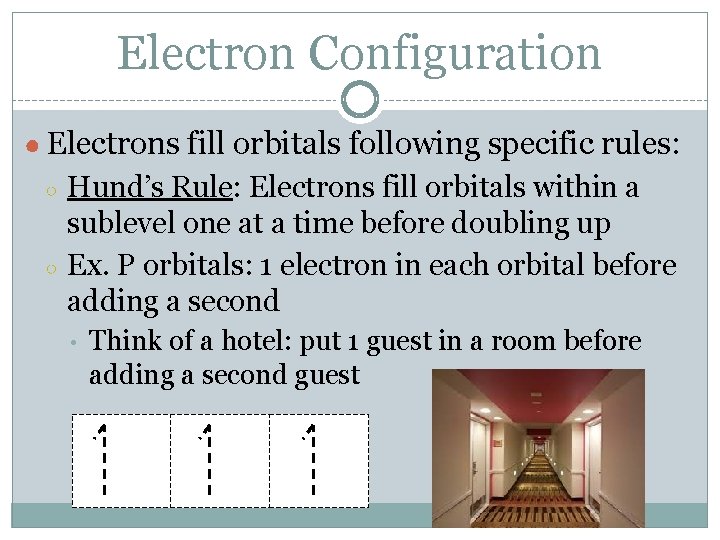
Electron Configuration ● Electrons fill orbitals following specific rules: ○ ○ Hund’s Rule: Electrons fill orbitals within a sublevel one at a time before doubling up Ex. P orbitals: 1 electron in each orbital before adding a second • Think of a hotel: put 1 guest in a room before adding a second guest

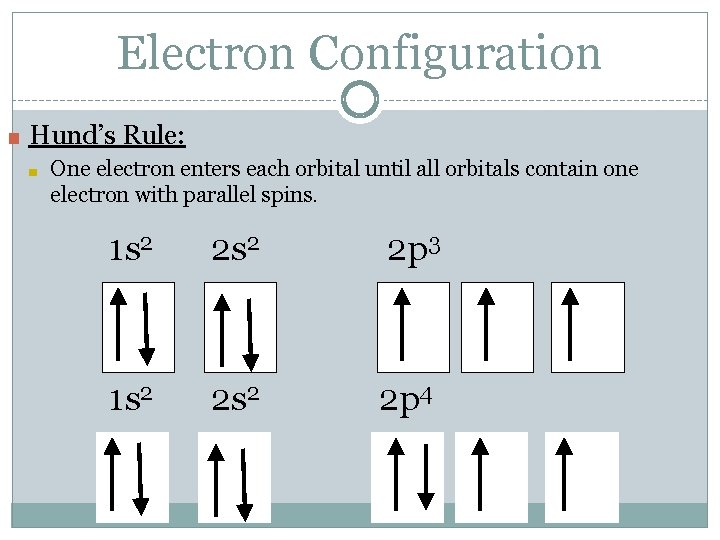
Electron Configuration ■ Hund’s Rule: ■ One electron enters each orbital until all orbitals contain one electron with parallel spins. 1 s 2 2 p 3 1 s 2 2 p 4

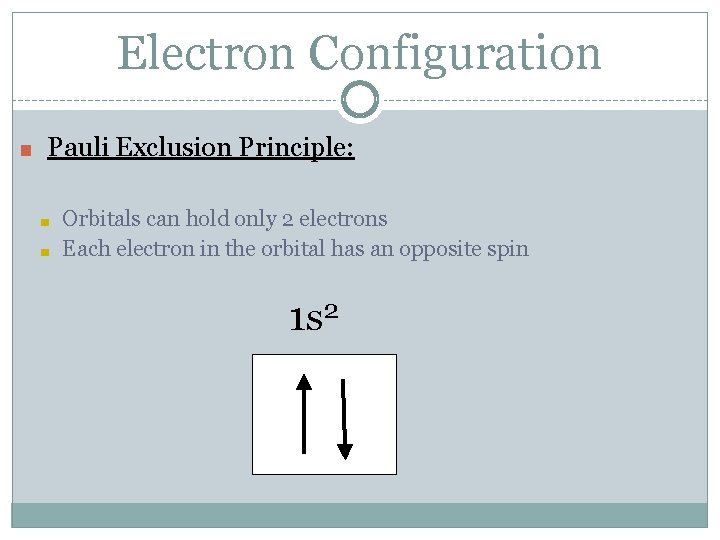
Electron Configuration ● Electrons fill orbitals following specific rules: ○ Pauli Exclusion Principal: There is a max of 2 electrons within each orbital and they must have equal and opposite spins • Think of a hotel: if you have to share a bed, sleep with one of your heads at the top and one at the bottom

Electron Configuration ■ Pauli Exclusion Principle: ■ ■ Orbitals can hold only 2 electrons Each electron in the orbital has an opposite spin 1 s 2

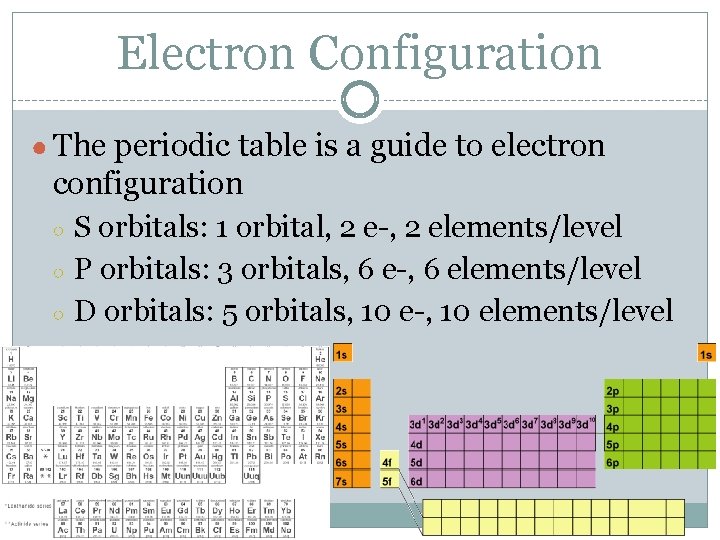
Electron Configuration ● The periodic table is a guide to electron configuration ○ ○ ○ S orbitals: 1 orbital, 2 e-, 2 elements/level P orbitals: 3 orbitals, 6 e-, 6 elements/level D orbitals: 5 orbitals, 10 e-, 10 elements/level

Electron Configuration ● Each energy level corresponds with the rows on the periodic table ○ Except!!! – D orbitals are 1 energy level lower than the row they are in • • • Sc, Ti, Cr, Mn… are n = 3 3 d is lower energy than 3 p Ex: Zn – 1 s 22 p 63 s 23 p 64 s 23 d 10

Electron Configuration ● Practice: ○ Sodium ○ Chlorine ○ Bromine ○ Zinc ○ Neon

Electron Configuration ● Nobel Gas e- configuration short hand: ○ Write the nobel gas that comes directly before the element in brackets first, then write the rest of the configuration • Ex. Ca – [Ar]2 s 2

Electron Configuration