Electron Configuration Atomic Properties and Periodic Trends Electron




![Cation Radii Na [Ne] 3 s 1 outer electron is shielded by Na+ [Ne] Cation Radii Na [Ne] 3 s 1 outer electron is shielded by Na+ [Ne]](https://slidetodoc.com/presentation_image_h2/58e9879e96ce16de646e0407bf0b6183/image-19.jpg)








![SOLUTION: (a) Mn 2+(Z = 25) Mn([Ar]4 s 23 d 5) (b) Cr 3+(Z SOLUTION: (a) Mn 2+(Z = 25) Mn([Ar]4 s 23 d 5) (b) Cr 3+(Z](https://slidetodoc.com/presentation_image_h2/58e9879e96ce16de646e0407bf0b6183/image-40.jpg)
- Slides: 40

Electron Configuration: Atomic Properties and Periodic Trends § Electron configurations (representation of atomic structures) help determine atomic and chemical properties. § Properties such as atomic radius, ionization energy, and metallic character and electron affinity are all periodic!

Summation of Periodic Trends

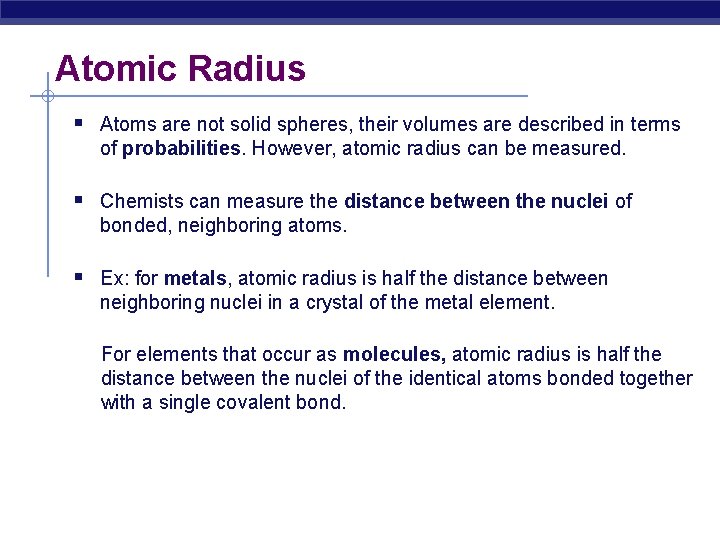
Atomic Radius § Atoms are not solid spheres, their volumes are described in terms of probabilities. However, atomic radius can be measured. § Chemists can measure the distance between the nuclei of bonded, neighboring atoms. § Ex: for metals, atomic radius is half the distance between neighboring nuclei in a crystal of the metal element. For elements that occur as molecules, atomic radius is half the distance between the nuclei of the identical atoms bonded together with a single covalent bond.

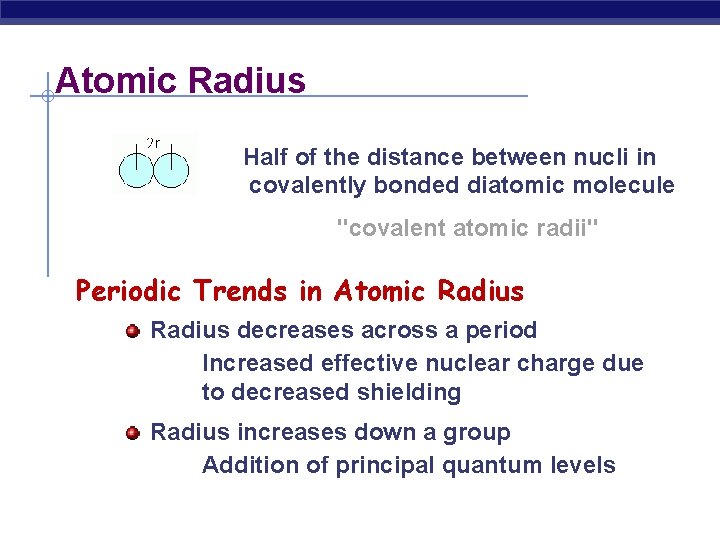
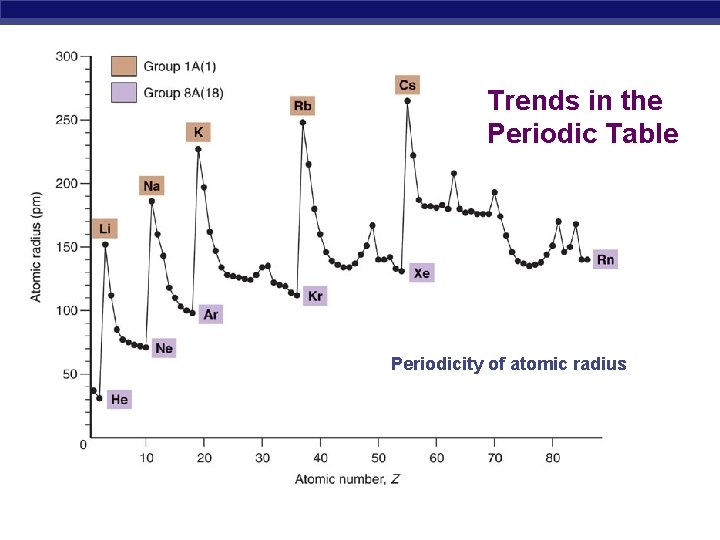
Atomic Radius Half of the distance between nucli in covalently bonded diatomic molecule "covalent atomic radii" Periodic Trends in Atomic Radius decreases across a period Increased effective nuclear charge due to decreased shielding Radius increases down a group Addition of principal quantum levels

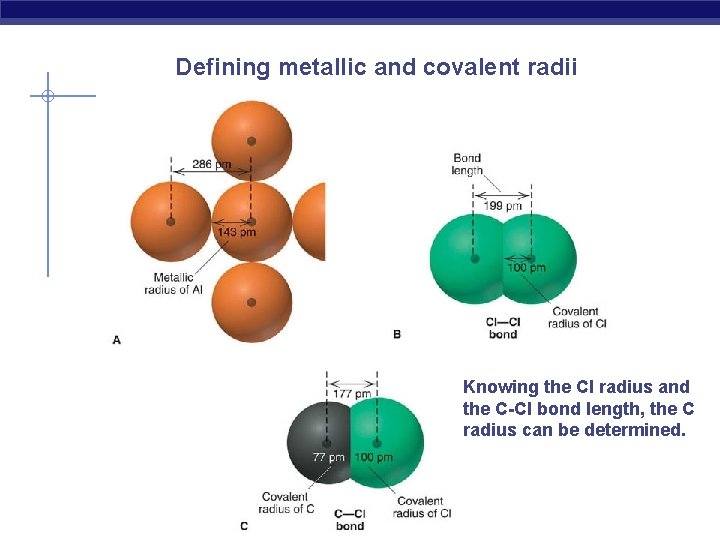
Defining metallic and covalent radii Knowing the Cl radius and the C-Cl bond length, the C radius can be determined.

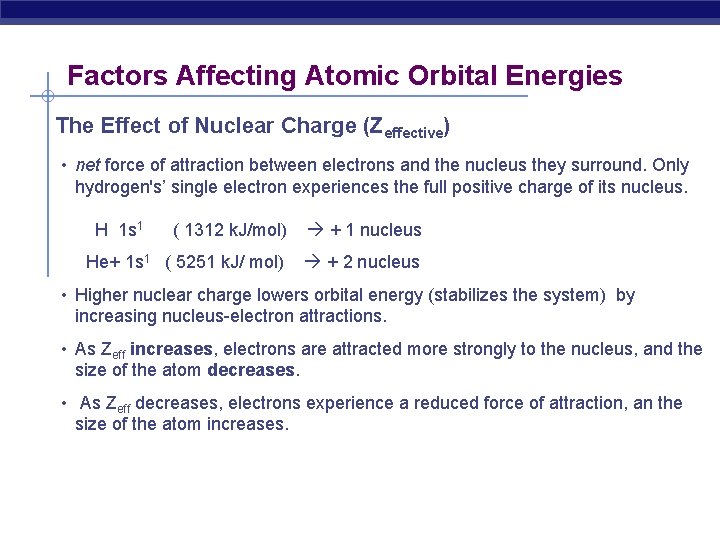
Factors Affecting Atomic Orbital Energies The Effect of Nuclear Charge (Zeffective) • net force of attraction between electrons and the nucleus they surround. Only hydrogen's’ single electron experiences the full positive charge of its nucleus. H 1 s 1 ( 1312 k. J/mol) + 1 nucleus He+ 1 s 1 ( 5251 k. J/ mol) + 2 nucleus • Higher nuclear charge lowers orbital energy (stabilizes the system) by increasing nucleus-electron attractions. • As Zeff increases, electrons are attracted more strongly to the nucleus, and the size of the atom decreases. • As Zeff decreases, electrons experience a reduced force of attraction, an the size of the atom increases.

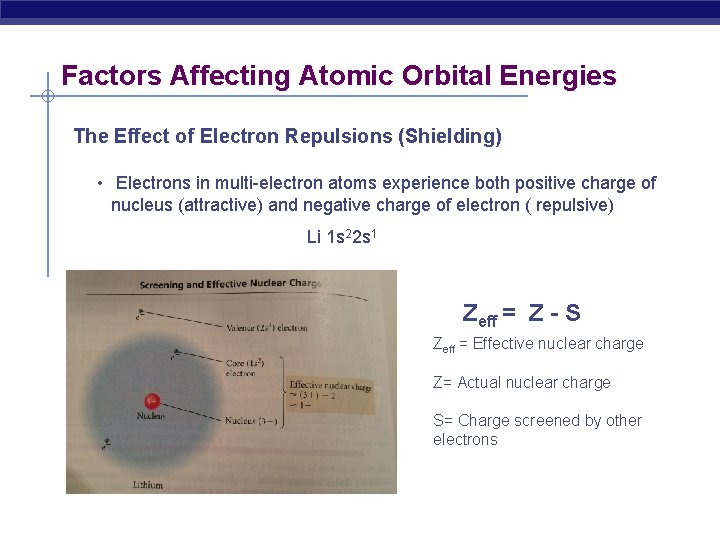
Factors Affecting Atomic Orbital Energies The Effect of Electron Repulsions (Shielding) • Electrons in multi-electron atoms experience both positive charge of nucleus (attractive) and negative charge of electron ( repulsive) Li 1 s 22 s 1 Zeff = Z - S Zeff = Effective nuclear charge Z= Actual nuclear charge S= Charge screened by other electrons

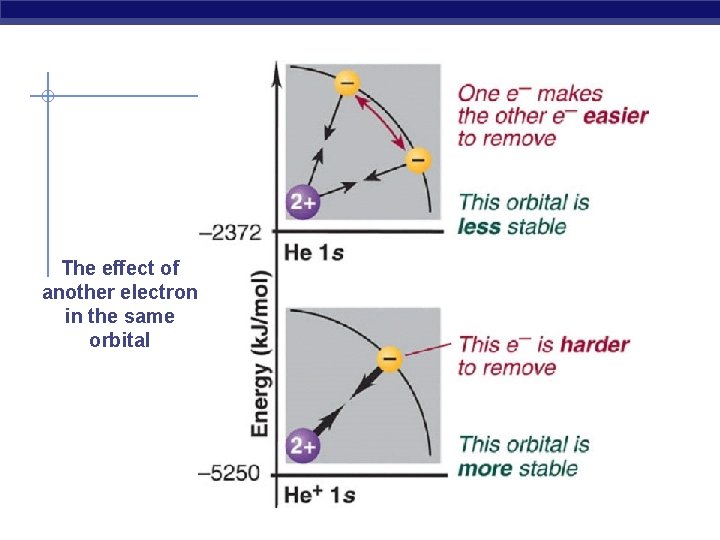
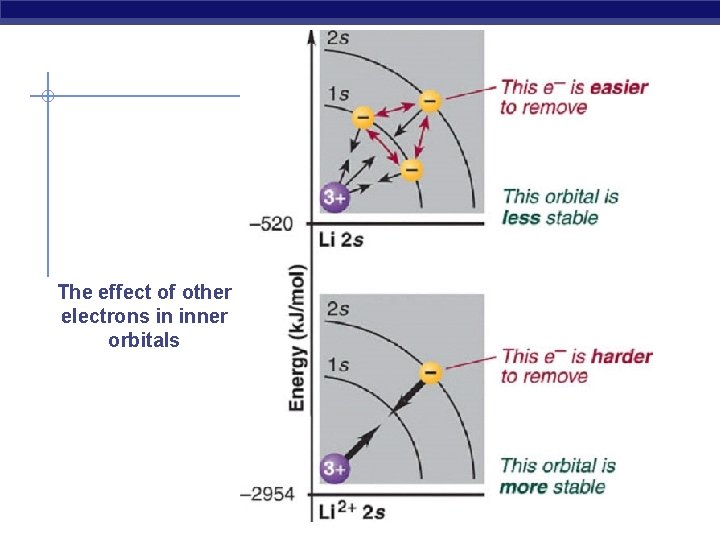
What happens to Beryllium ? Be 1 s 22 s 2 …. There are two types of shielding - the shielding of the outermost electrons by the core electrons - the shielding of the outermost electrons by each other Core electrons efficiently shield electrons in the outermost principal energy level from nuclear charge, but outermost electrons do not efficiently shield one another from nuclear charge. -the shielding experienced by any one of the outermost electrons due to the core electrons in nearly 2 - Shielding due to the outermost electrons is nearly zero

The effect of another electron in the same orbital

The effect of other electrons in inner orbitals

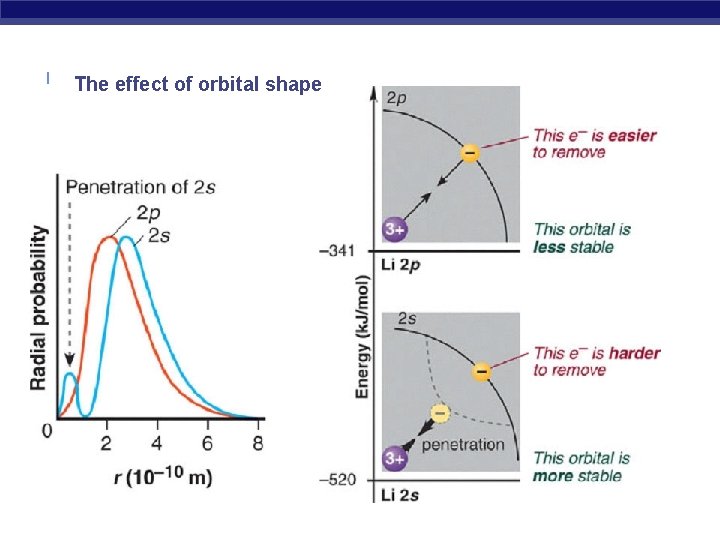
The effect of orbital shape

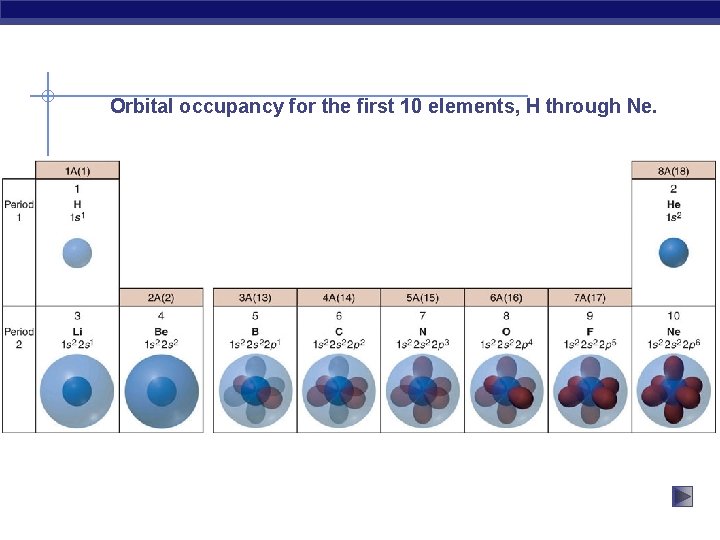
Orbital occupancy for the first 10 elements, H through Ne.

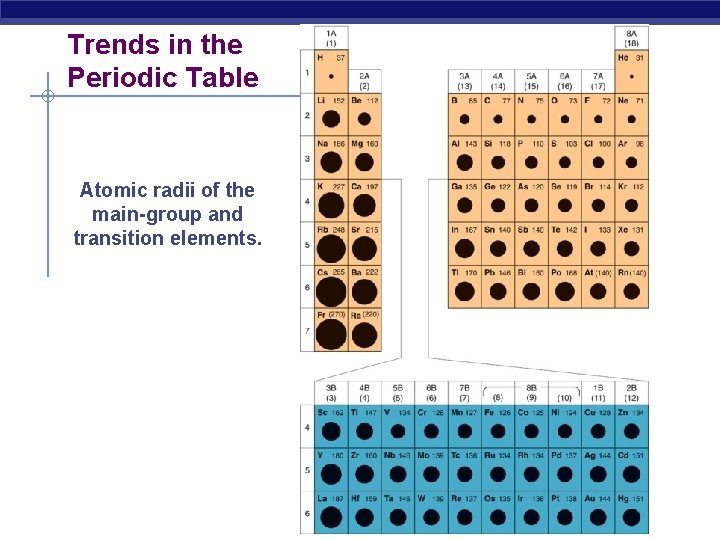
Trends in the Periodic Table Atomic radii of the main-group and transition elements.

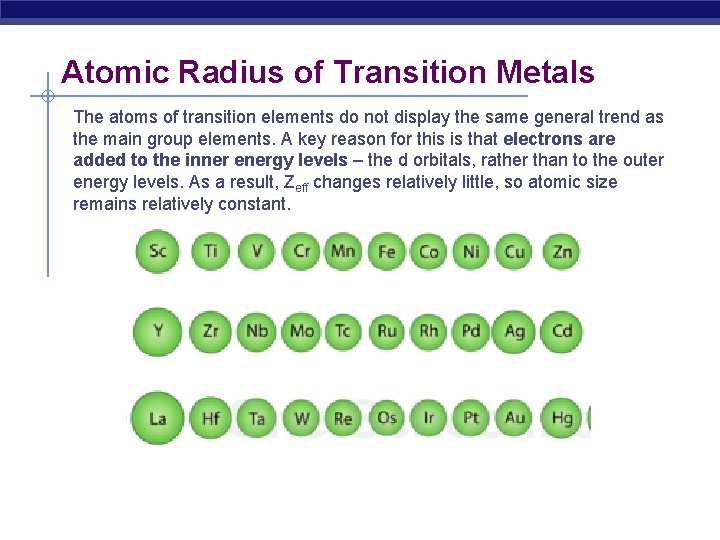
Atomic Radius of Transition Metals The atoms of transition elements do not display the same general trend as the main group elements. A key reason for this is that electrons are added to the inner energy levels – the d orbitals, rather than to the outer energy levels. As a result, Zeff changes relatively little, so atomic size remains relatively constant.

Trends in the Periodic Table Periodicity of atomic radius

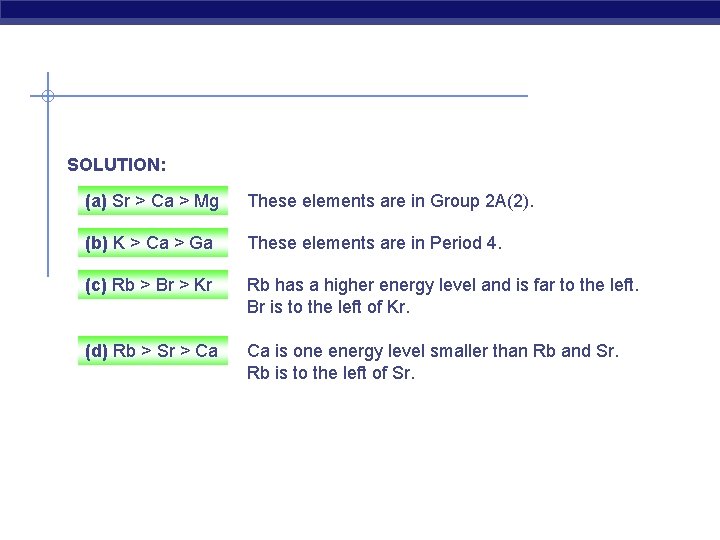
Ranking Elements by Atomic Size PROBLEM: Using only the periodic table rank each set of main group elements in order of decreasing atomic size: (a) Ca, Mg, Sr PLAN: (b) K, Ga, Ca (c) Br, Rb, Kr (d) Sr, Ca, Rb Elements in the same group decrease in size as you go up; elements decrease in size as you go across a period.

SOLUTION: (a) Sr > Ca > Mg These elements are in Group 2 A(2). (b) K > Ca > Ga These elements are in Period 4. (c) Rb > Br > Kr Rb has a higher energy level and is far to the left. Br is to the left of Kr. (d) Rb > Sr > Ca Ca is one energy level smaller than Rb and Sr. Rb is to the left of Sr.

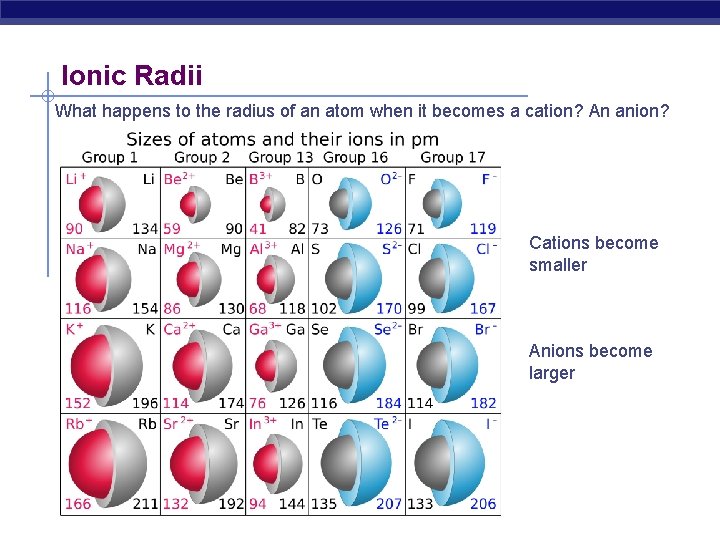
Ionic Radii What happens to the radius of an atom when it becomes a cation? An anion? Cations become smaller Anions become larger
![Cation Radii Na Ne 3 s 1 outer electron is shielded by Na Ne Cation Radii Na [Ne] 3 s 1 outer electron is shielded by Na+ [Ne]](https://slidetodoc.com/presentation_image_h2/58e9879e96ce16de646e0407bf0b6183/image-19.jpg)
Cation Radii Na [Ne] 3 s 1 outer electron is shielded by Na+ [Ne] lost outermost electron (SMALLER) Anion Radii Cl [Ne] 3 s 23 p 5 Cl- [Ne] 3 s 23 p 6 additional electron, but no additional proton to increase nuclear charge (LARGER)

Observing Ions S 2 - (184 pm) 18 e 16 protons Cl- (181 pm) 18 e 17 protons K+ (133 pm) 18 e 18 protons Ca 2+ (99 pm) 18 e 19 protons

Ranking Ions by Size PROBLEM: Rank each set of ions in order of decreasing size, and explain your ranking: (a) Ca 2+, Sr 2+, Mg 2+ PLAN: (b) K+, S 2 -, Cl - (c) Au+, Au 3+ Compare positions in the periodic table, formation of positive and negative ions and changes in size due to gain or loss of electrons.

SOLUTION: (a) Sr 2+ > Ca 2+ > Mg 2+ (b) S 2 - > Cl - > K+ (c) Au+ > Au 3+ These are members of the same Group (2 A/2) and therefore decrease in size going up the group. The ions are isoelectronic; S 2 - has the smallest Zeff and therefore is the largest while K+ is a cation with a large Zeff and is the smallest. The higher the + charge, the smaller the ion.

Ionization Energy the energy required to remove an electron from an atom ( always +) Increases for successive electrons taken from the same atom Tends to increase across a period • Electrons in the same quantum level do not shield as effectively as electrons in inner levels • Irregularities at half filled and filled sublevels due to extra repulsion of electrons paired in orbitals, making them easier to remove Tends to decrease down a group • Outer electrons are farther from the nucleus

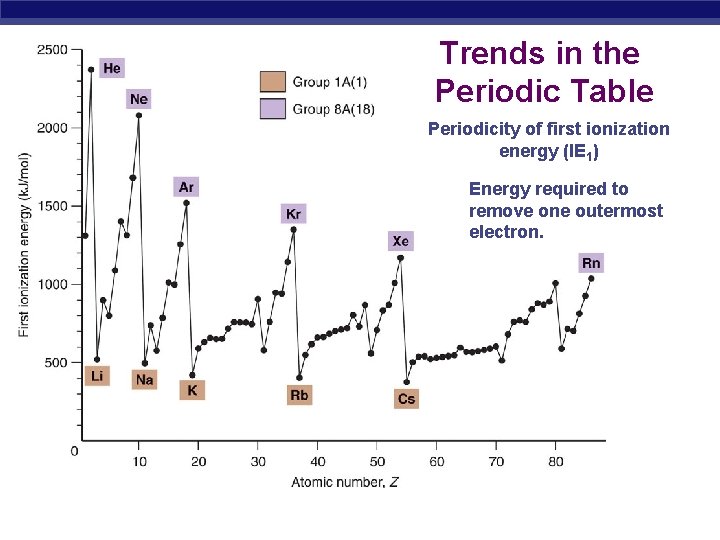
Trends in the Periodic Table Periodicity of first ionization energy (IE 1) Energy required to remove one outermost electron.

First Ionization Energies • Ionization energy decreases down a group because Zeff decreases down a group. As n increases, the orbitals become larger and electrons in the outermost principal level are farther away from the positively charged nucleus. • Ionization every increase across a period because the outermost principal energy level generally experiences a greater Zeff

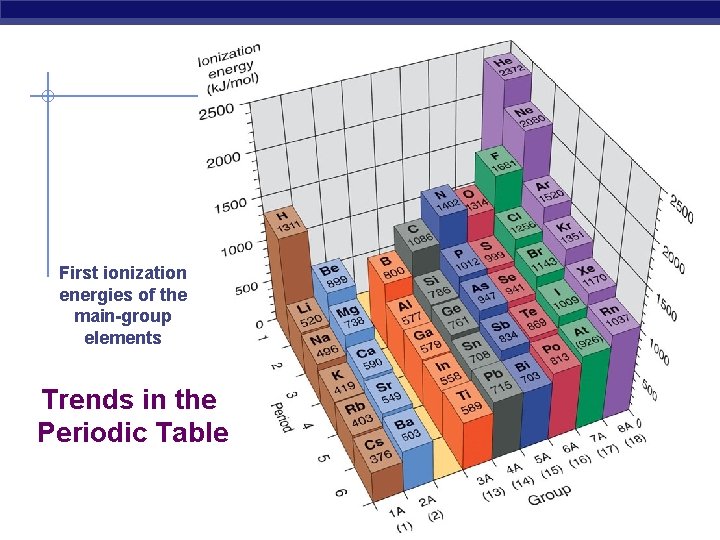
First ionization energies of the main-group elements Trends in the Periodic Table

• The drop of IE 1 with group 13 occurs because electrons start to fill the np orbitals, which are higher in energy than ns orbitals, so the additional electron is more easily removed • Group 16 marks the pairing of the first pairing of the p orbital electrons. The np 3 configuration of nitrogen is more stable than the np 4 configuration of oxygen • Electron repulsions increase the orbital energy in oxygen, therefore less energy is required to remove that electron.

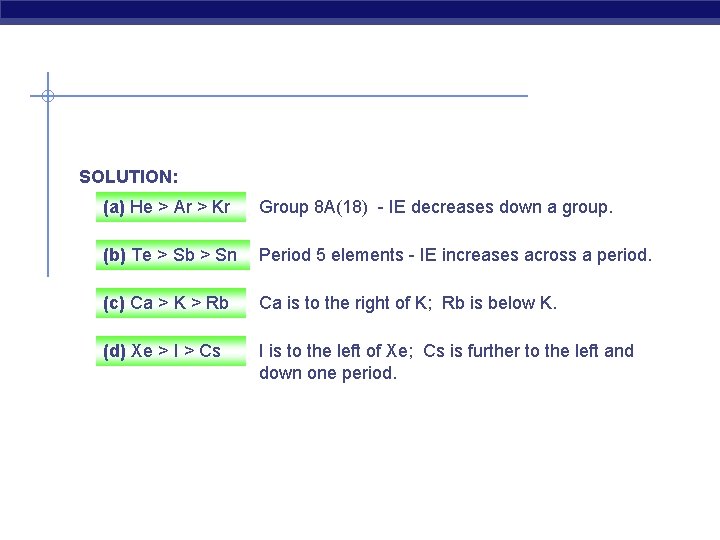
Ranking Elements by First Ionization Energy PROBLEM: (a) Kr, He, Ar Using the periodic table only, rank the elements in each of the following sets in order of decreasing IE 1: (b) Sb, Te, Sn (c) K, Ca, Rb (d) I, Xe, Cs

SOLUTION: (a) He > Ar > Kr Group 8 A(18) - IE decreases down a group. (b) Te > Sb > Sn Period 5 elements - IE increases across a period. (c) Ca > K > Rb Ca is to the right of K; Rb is below K. (d) Xe > I > Cs I is to the left of Xe; Cs is further to the left and down one period.

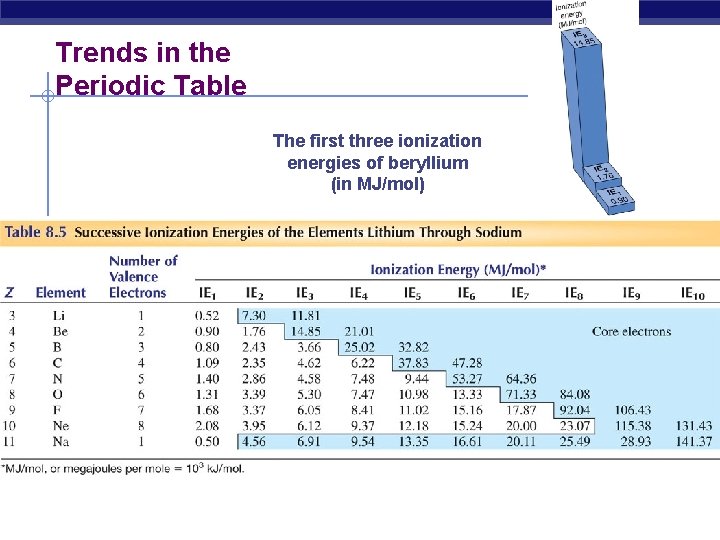
Trends in the Periodic Table The first three ionization energies of beryllium (in MJ/mol)

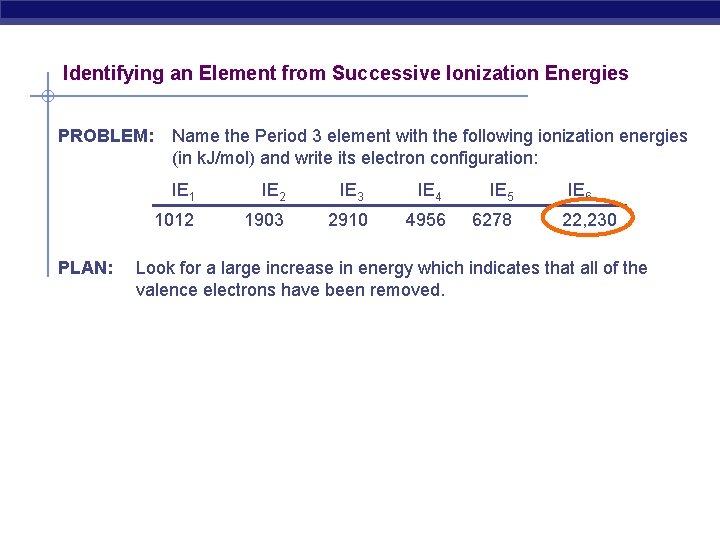
Identifying an Element from Successive Ionization Energies PROBLEM: PLAN: Name the Period 3 element with the following ionization energies (in k. J/mol) and write its electron configuration: IE 1 IE 2 IE 3 IE 4 IE 5 1012 1903 2910 4956 6278 IE 6 22, 230 Look for a large increase in energy which indicates that all of the valence electrons have been removed.

SOLUTION: The largest increase occurs after IE 5, that is, after the 5 th valence electron has been removed. Five electrons would mean that the valence configuration is 3 s 23 p 3 and the element must be phosphorous, P (Z = 15). The complete electron configuration is 1 s 22 p 63 s 23 p 3.

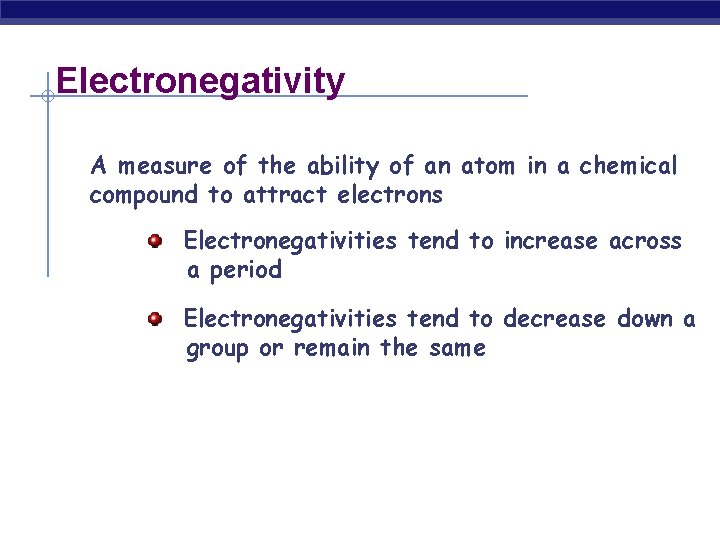
Electronegativity A measure of the ability of an atom in a chemical compound to attract electrons Electronegativities tend to increase across a period Electronegativities tend to decrease down a group or remain the same

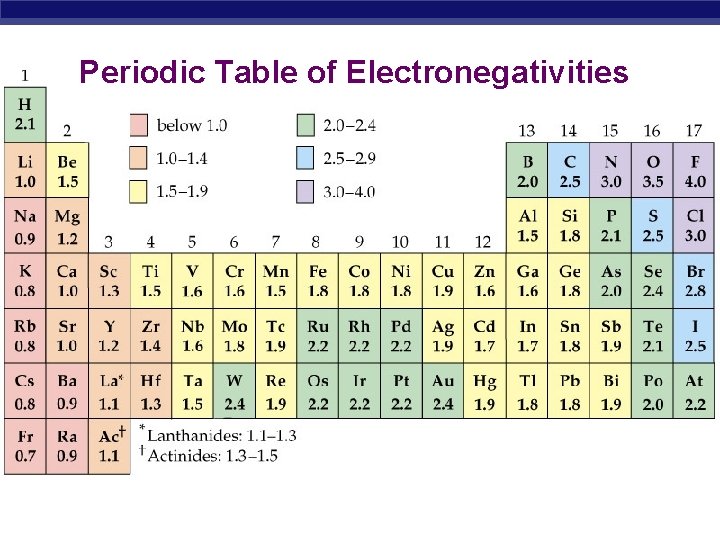
Periodic Table of Electronegativities

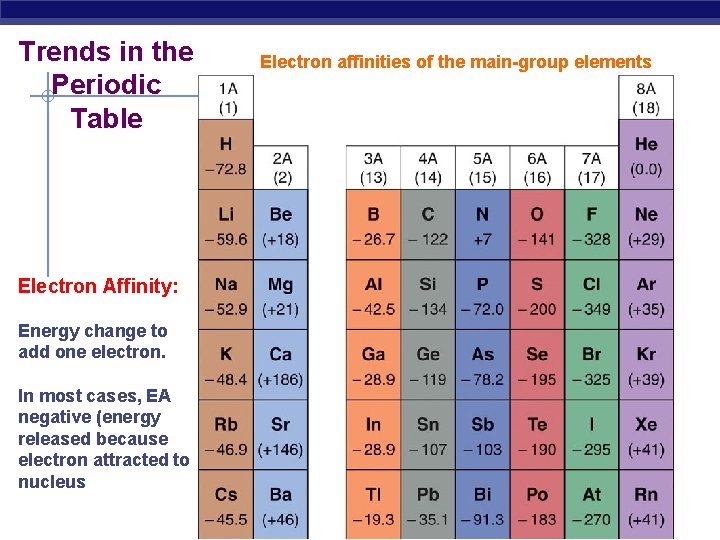
Trends in the Periodic Table Electron Affinity: Energy change to add one electron. In most cases, EA negative (energy released because electron attracted to nucleus Electron affinities of the main-group elements

• Most groups of the periodic table do not exhibit any definite trend in electron affinity (except Group 1) • Electron affinity generally becomes more negative as we move to the right across a period • The halogens have the most negative electron affinities because they have a configuration of ns 2 np 5, which means they obtain a noble gas configuration with extra electron.

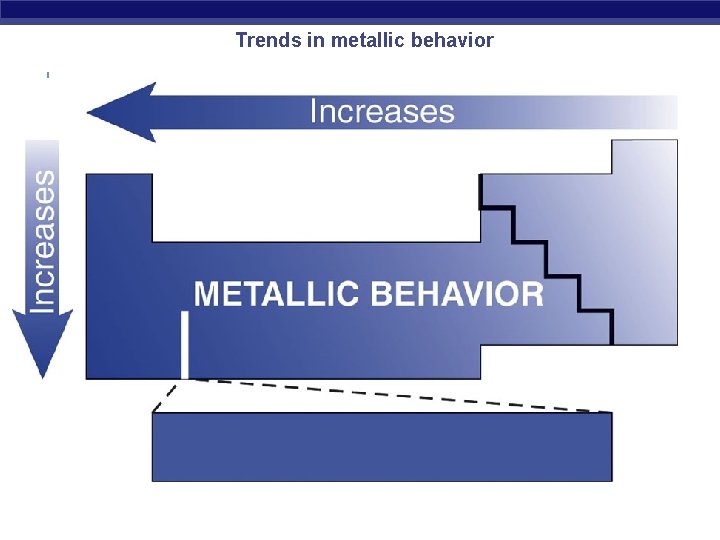
Trends in metallic behavior

Magnetic Properties of Transition Metal Ions A species with unpaired electrons exhibits paramagnetism. It is attracted by an external magnetic field. Ag [Kr] 5 s 14 d 10 Species with all paired e’s, not attracted. . . . diamagnetic Zn [Ar] 4 s 23 d 10

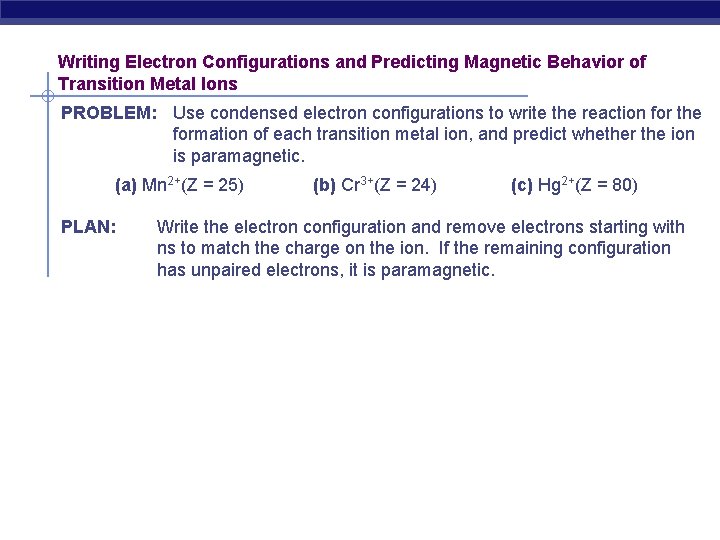
Writing Electron Configurations and Predicting Magnetic Behavior of Transition Metal Ions PROBLEM: Use condensed electron configurations to write the reaction for the formation of each transition metal ion, and predict whether the ion is paramagnetic. (a) Mn 2+(Z = 25) PLAN: (b) Cr 3+(Z = 24) (c) Hg 2+(Z = 80) Write the electron configuration and remove electrons starting with ns to match the charge on the ion. If the remaining configuration has unpaired electrons, it is paramagnetic.
![SOLUTION a Mn 2Z 25 MnAr4 s 23 d 5 b Cr 3Z SOLUTION: (a) Mn 2+(Z = 25) Mn([Ar]4 s 23 d 5) (b) Cr 3+(Z](https://slidetodoc.com/presentation_image_h2/58e9879e96ce16de646e0407bf0b6183/image-40.jpg)
SOLUTION: (a) Mn 2+(Z = 25) Mn([Ar]4 s 23 d 5) (b) Cr 3+(Z = 24) Cr([Ar]4 s 23 d 6) (c) Hg 2+(Z = 80) Hg([Xe]6 s 24 f 145 d 10) Mn 2+ ([Ar] 3 d 5) + 2 e. Cr 3+ ([Ar] 3 d 5) + 3 e- paramagnetic Hg 2+ ([Xe] 4 f 145 d 10) + 2 enot paramagnetic (is diamagnetic)