Electron Configuration Assigning Electrons to Orbitals Review of

Electron Configuration Assigning Electrons to Orbitals
Review of Dorm Room Activity. . . • Different types of rooms (s, p, d, and f) • Students prefer to live on lower floors because there is no elevator in the building • Two students per dorm room
Electron Configuration Analogy • Different shapes of electron shells • Electrons prefer to be close to the nucleus at lower energy levels – Inside shell (1 s) is the ground state – Electrons are attracted to the positive protons inside the nucleus, which is why they like being close • Two electrons per orbital (each 'floor' of the dorm room is a shell, each 'room' is an orbital)
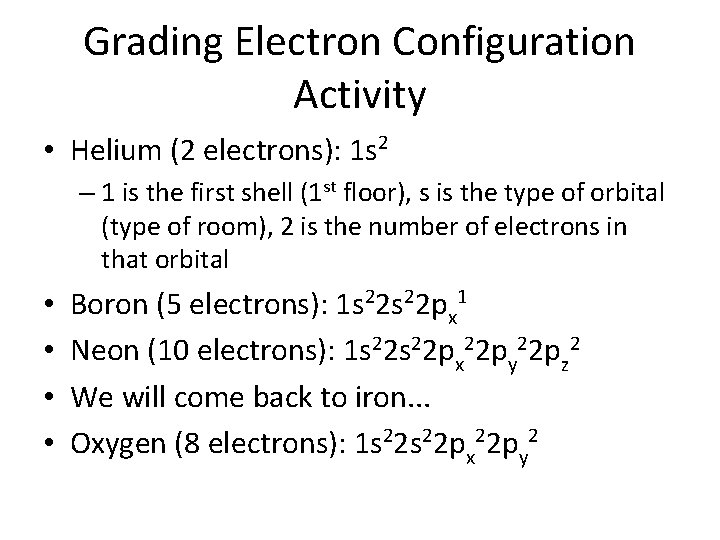
Grading Electron Configuration Activity • Helium (2 electrons): 1 s 2 – 1 is the first shell (1 st floor), s is the type of orbital (type of room), 2 is the number of electrons in that orbital • • Boron (5 electrons): 1 s 22 px 1 Neon (10 electrons): 1 s 22 px 22 py 22 pz 2 We will come back to iron. . . Oxygen (8 electrons): 1 s 22 px 22 py 2
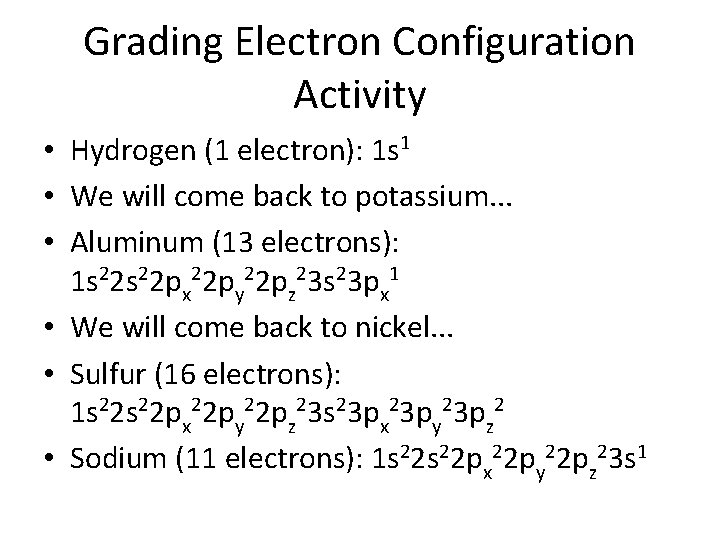
Grading Electron Configuration Activity • Hydrogen (1 electron): 1 s 1 • We will come back to potassium. . . • Aluminum (13 electrons): 1 s 22 px 22 py 22 pz 23 s 23 px 1 • We will come back to nickel. . . • Sulfur (16 electrons): 1 s 22 px 22 py 22 pz 23 s 23 px 23 py 23 pz 2 • Sodium (11 electrons): 1 s 22 px 22 py 22 pz 23 s 1

Additional Rule. . . • In the dorm room activity, our additional rule was that students would prefer to be in a nicer room on a higher floor (4 s instead of 3 d), so we had to fill the s rooms on the next floor before coming back to the d rooms on the lower floor.
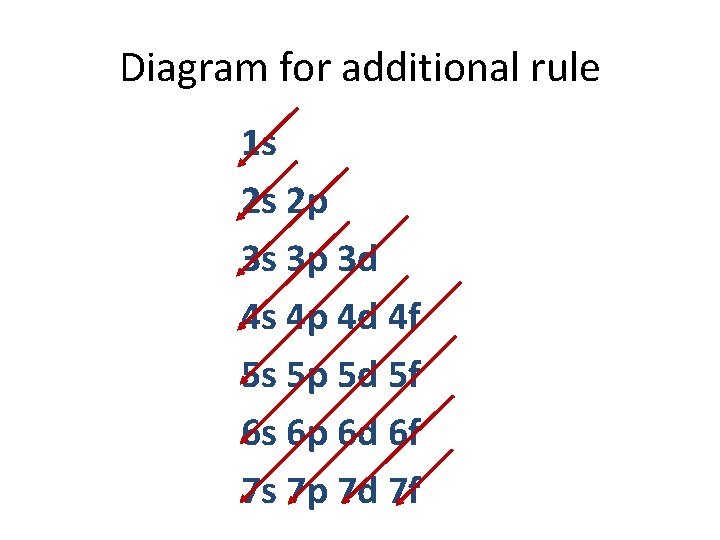
Diagram for additional rule 1 s 2 s 2 p 3 s 3 p 3 d 4 s 4 p 4 d 4 f 5 s 5 p 5 d 5 f 6 s 6 p 6 d 6 f 7 s 7 p 7 d 7 f
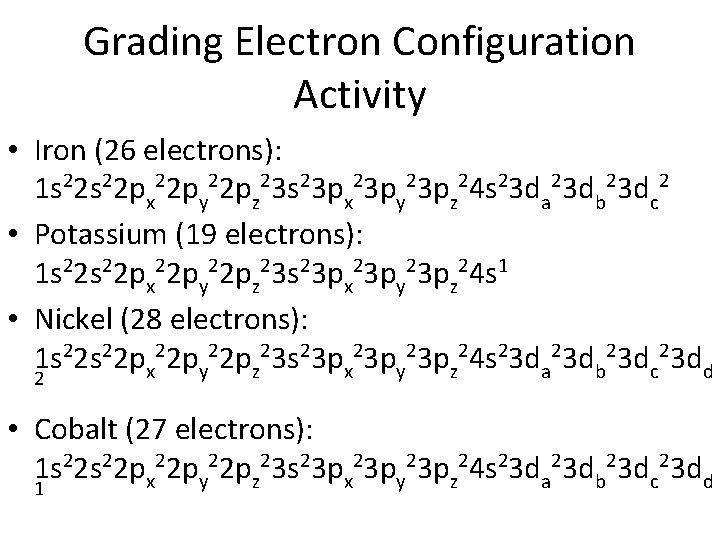
Grading Electron Configuration Activity • Iron (26 electrons): 1 s 22 px 22 py 22 pz 23 s 23 px 23 py 23 pz 24 s 23 da 23 db 23 dc 2 • Potassium (19 electrons): 1 s 22 px 22 py 22 pz 23 s 23 px 23 py 23 pz 24 s 1 • Nickel (28 electrons): 1 s 22 px 22 py 22 pz 23 s 23 px 23 py 23 pz 24 s 23 da 23 db 23 dc 23 dd 2 • Cobalt (27 electrons): 1 s 22 px 22 py 22 pz 23 s 23 px 23 py 23 pz 24 s 23 da 23 db 23 dc 23 dd 1
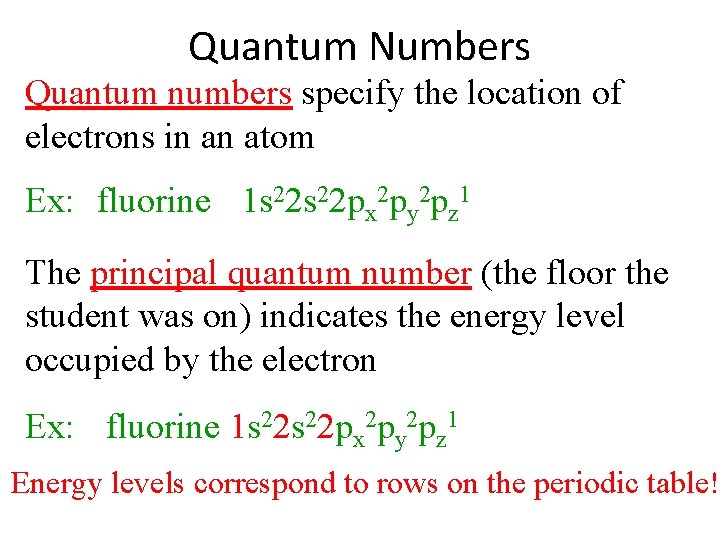
Quantum Numbers Quantum numbers specify the location of electrons in an atom Ex: fluorine 1 s 22 px 2 py 2 pz 1 The principal quantum number (the floor the student was on) indicates the energy level occupied by the electron Ex: fluorine 1 s 22 px 2 py 2 pz 1 Energy levels correspond to rows on the periodic table!
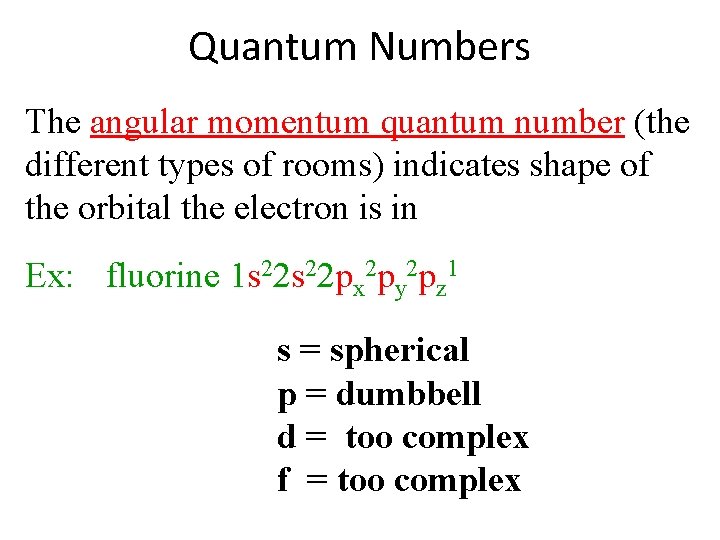
Quantum Numbers The angular momentum quantum number (the different types of rooms) indicates shape of the orbital the electron is in Ex: fluorine 1 s 22 px 2 py 2 pz 1 s = spherical p = dumbbell d = too complex f = too complex
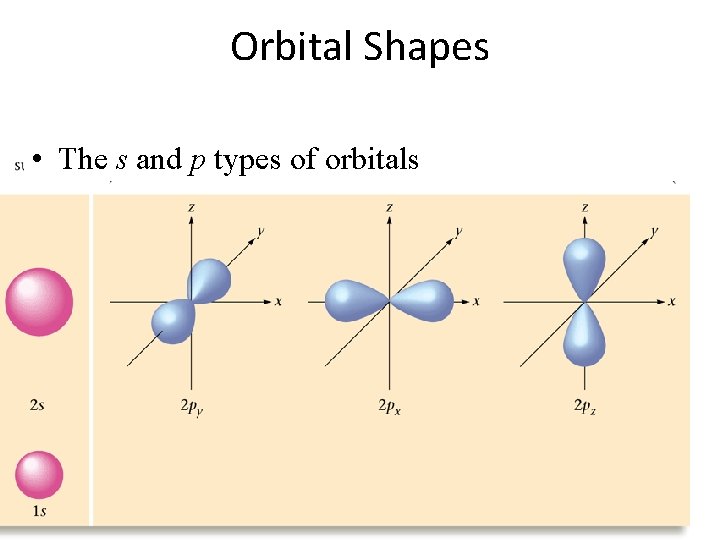
Orbital Shapes • The s and p types of orbitals
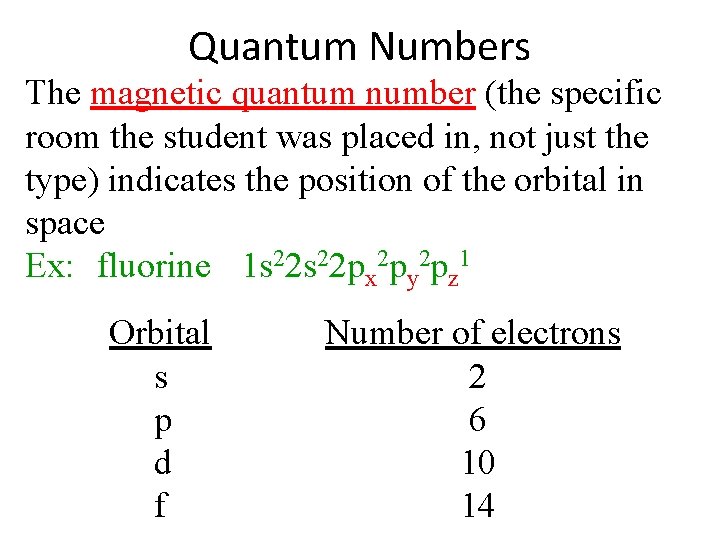
Quantum Numbers The magnetic quantum number (the specific room the student was placed in, not just the type) indicates the position of the orbital in space Ex: fluorine 1 s 22 px 2 py 2 pz 1 Orbital s p d f Number of electrons 2 6 10 14
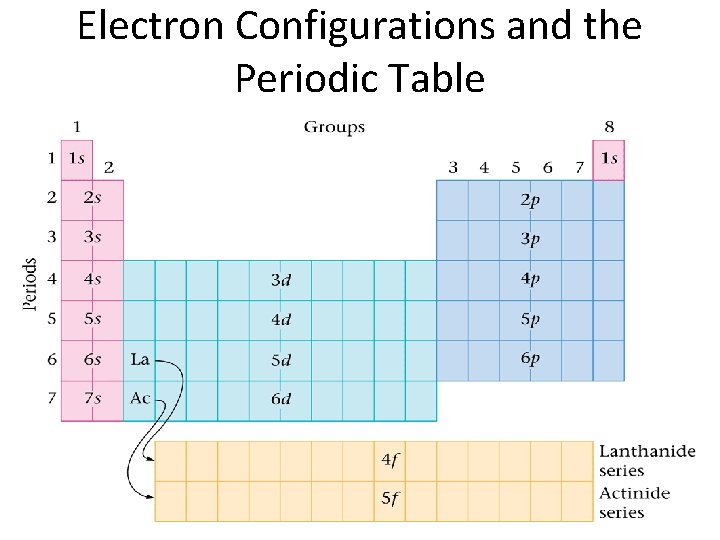
Electron Configurations and the Periodic Table
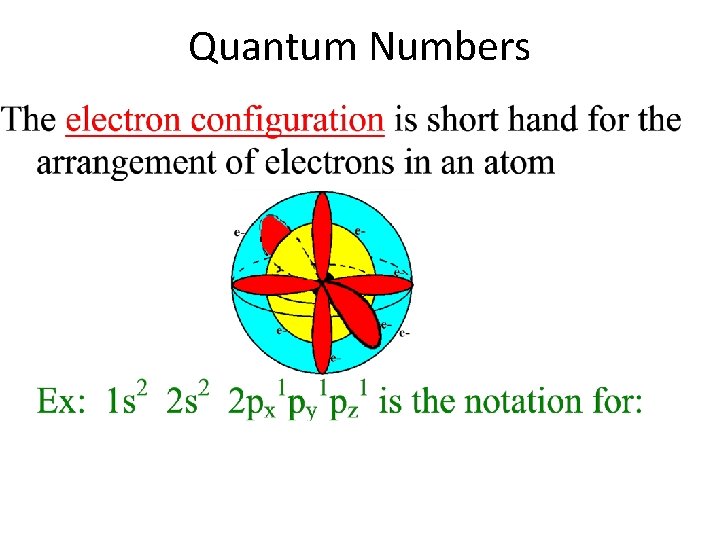
Quantum Numbers
- Slides: 14