ELECTRON CLOUDS TUTORIAL FROM QUANTUM THEORY TO ELECTRON


- Slides: 20

ELECTRON CLOUDS TUTORIAL FROM QUANTUM THEORY TO ELECTRON CONFIGURATIONS

MAIN MENU QUANTUM THEORY ORBITALS HOW ARE ORBITALS FORMED? HOW ARE ORBITALS FILLED? QUANTUM NUMBERS ELECTRONIC CONFIGURATIONS

QUANTUM THEORY �Bohr ◦ Electrons are in orbits around the nucleus like the solar system �De Broglie ◦ Electrons have particle and wave properties. Next

QUANTUM THEORY �Heisenberg ◦ You can’t know at a particular time where an electron is and where is it heading. �Schrödinger ◦ Proved and described electron wavelike behavior

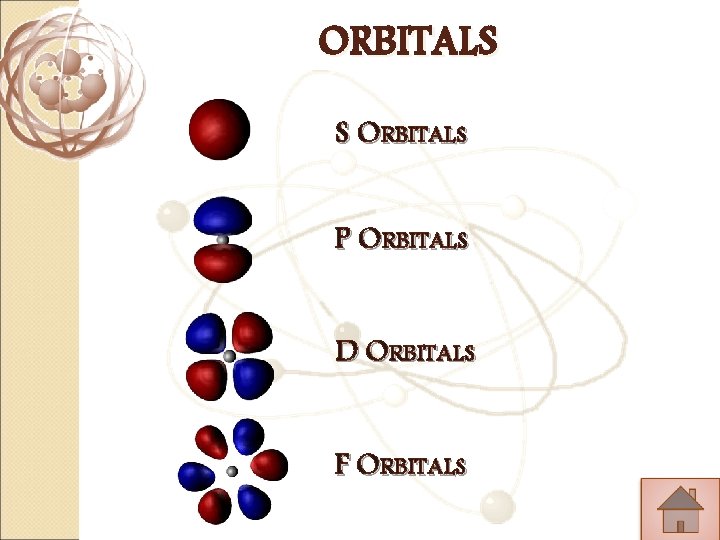
ORBITALS S ORBITALS P ORBITALS D ORBITALS F ORBITALS

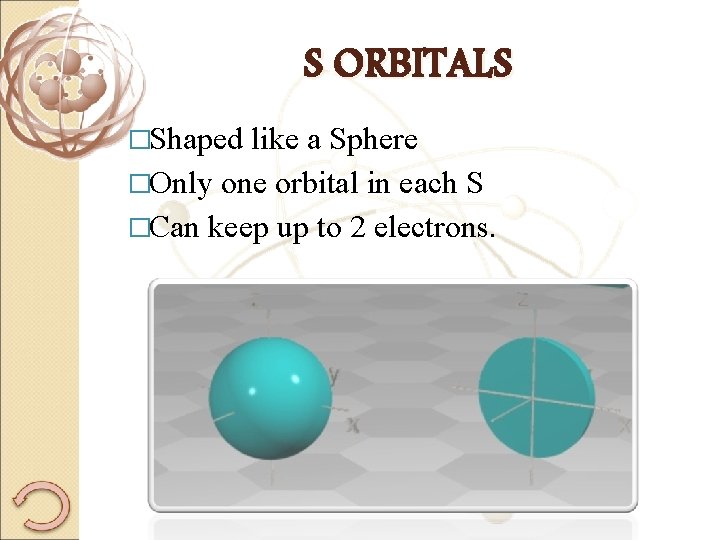
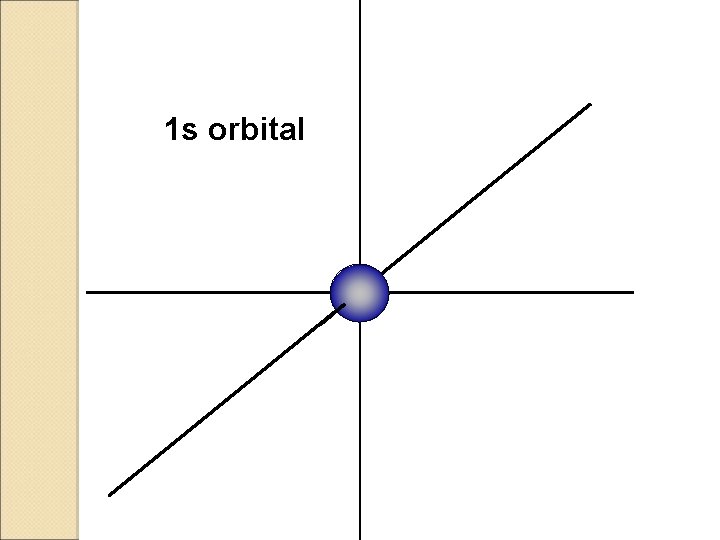
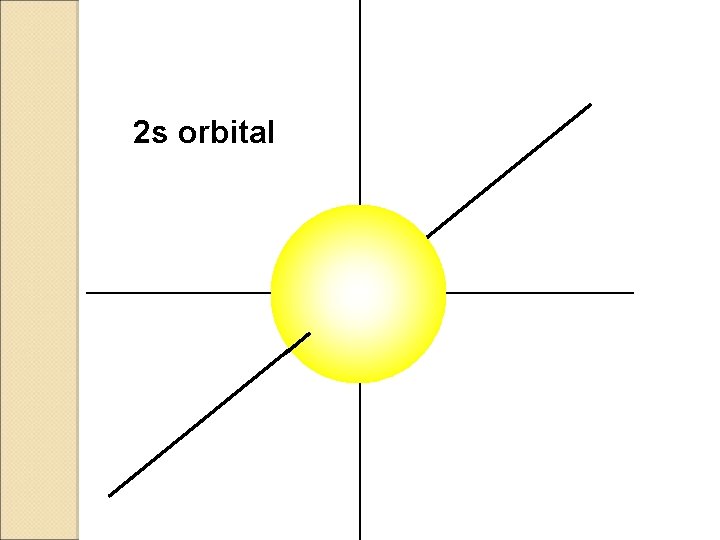
S ORBITALS �Shaped like a Sphere �Only one orbital in each S �Can keep up to 2 electrons.

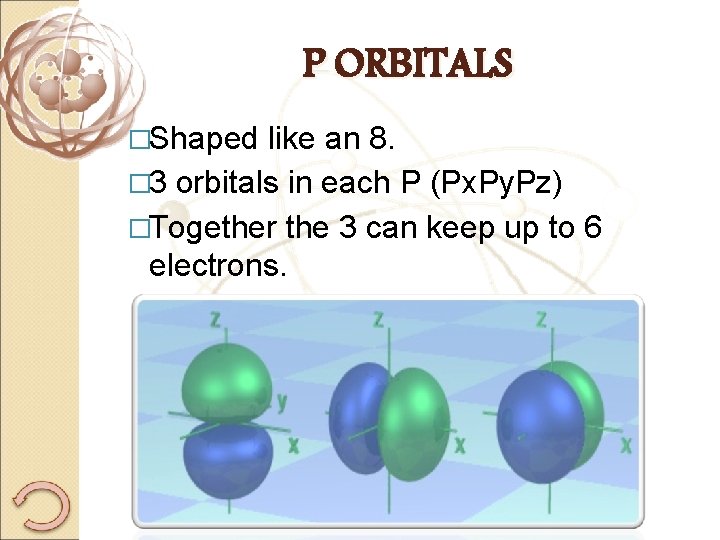
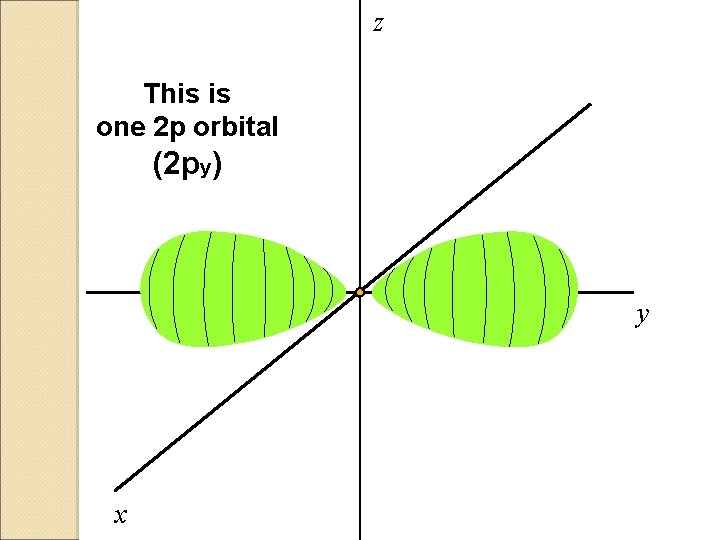
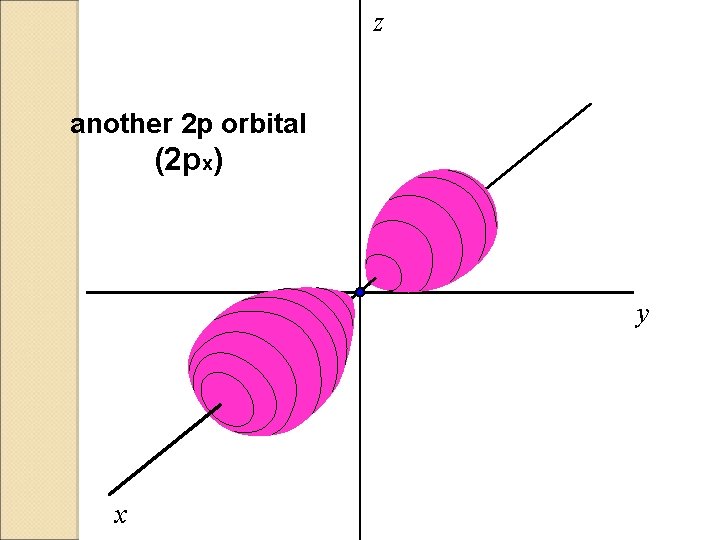
P ORBITALS �Shaped like an 8. � 3 orbitals in each P (Px. Py. Pz) �Together the 3 can keep up to 6 electrons.

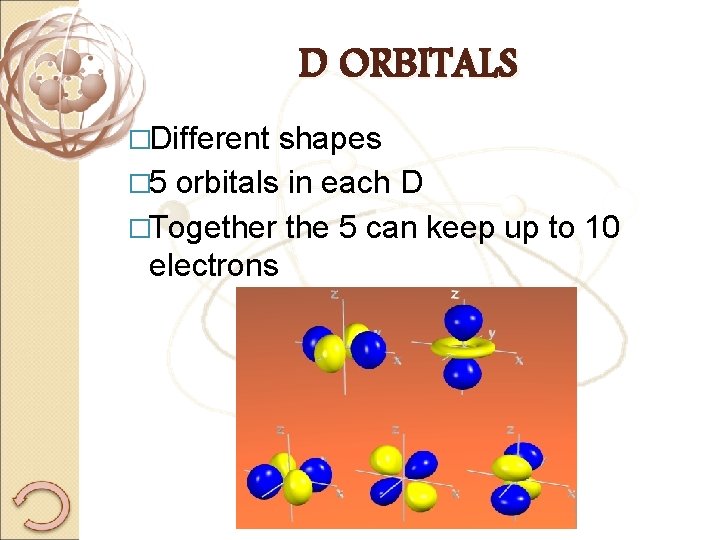
D ORBITALS �Different shapes � 5 orbitals in each D �Together the 5 can keep up to 10 electrons

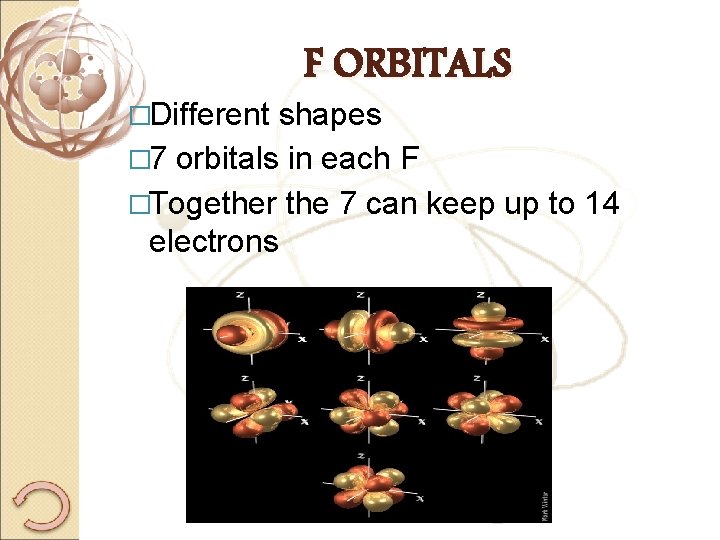
�Different F ORBITALS shapes � 7 orbitals in each F �Together the 7 can keep up to 14 electrons

HOW YOU FORM ORBITALS? �The next slides tell you how s and p orbitals are drawn.

1 s orbital

2 s orbital

z This is one 2 p orbital (2 py) y x

z another 2 p orbital (2 px) y x

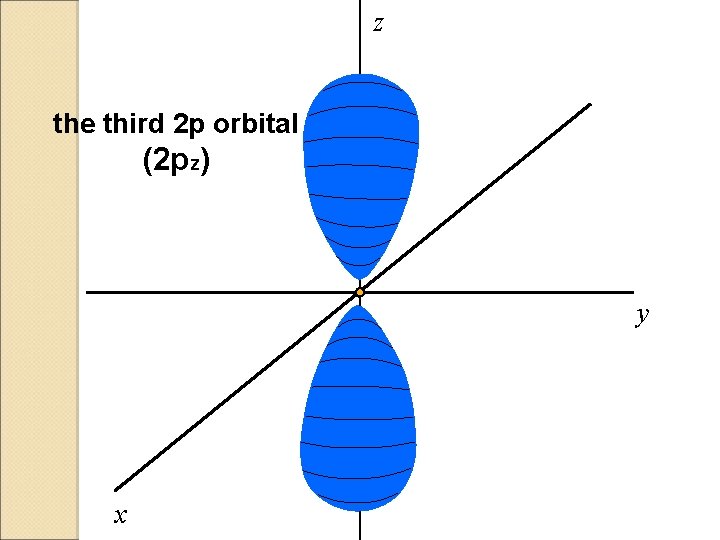
z the third 2 p orbital (2 pz) y x

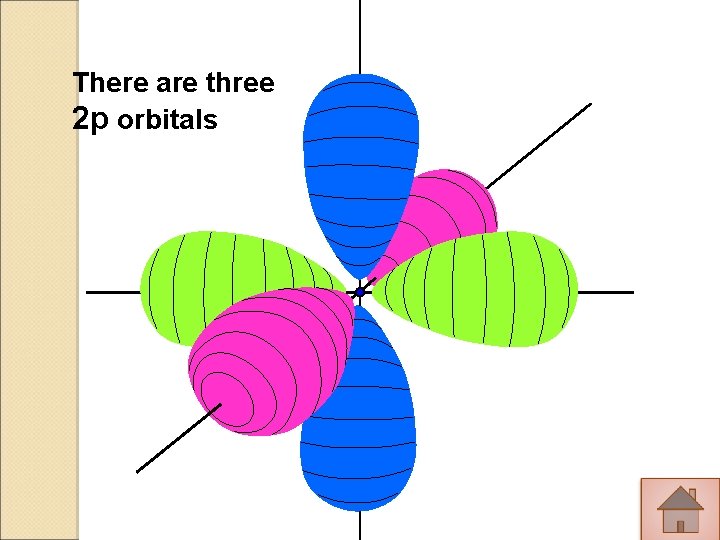
There are three 2 p orbitals

HOW DO ORBITALS FILL? �The next slides show you how orbitals fill in an element configuration.

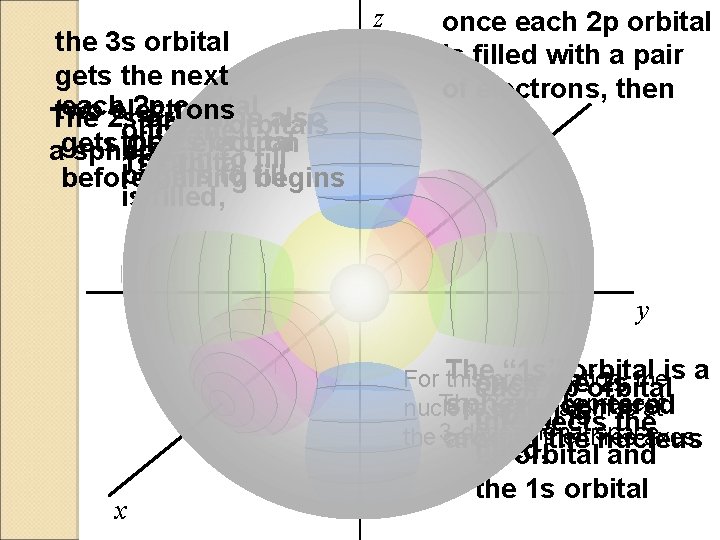
the 3 s orbital gets the next each 2 p orbital two electrons The 2 s orbital is also the 2 p orbitals once the 2 selectron orbital one agets sphere. begin to fill 1 s orbital begins to fill before pairing begins is filled, z once each 2 p orbital is filled with a pair of electrons, then y “ 1 s”the orbital is a For The thisonce presentation, the 2 s each 2 p orbital The 3 axes represent sphere, centered nucleus of the atom is at orbital is intersects the 3 -dimensional space the center of the axes. around thethree nucleus x filled, 2 s orbital and the 1 s orbital

z y x

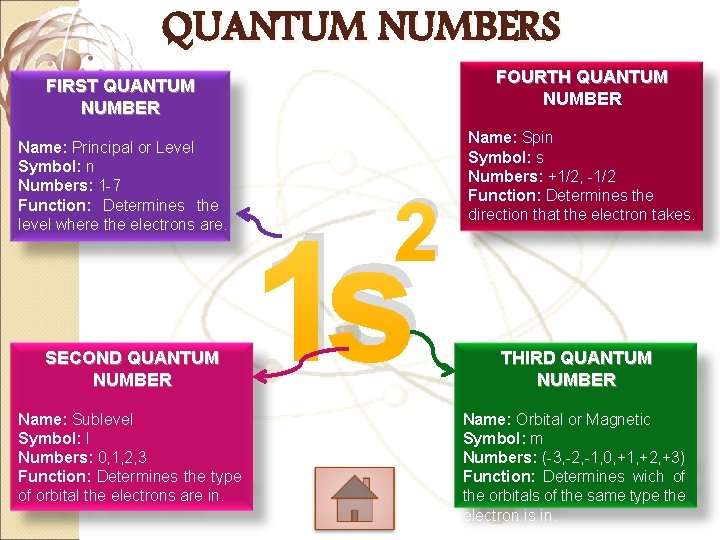
QUANTUM NUMBERS FOURTH QUANTUM NUMBER FIRST QUANTUM NUMBER Name: Principal or Level Symbol: n Numbers: 1 -7 Function: Determines the level where the electrons are. SECOND QUANTUM NUMBER Name: Sublevel Symbol: l Numbers: 0, 1, 2, 3 Function: Determines the type of orbital the electrons are in. 2 1 s Name: Spin Symbol: s Numbers: +1/2, -1/2 Function: Determines the direction that the electron takes. THIRD QUANTUM NUMBER Name: Orbital or Magnetic Symbol: m Numbers: (-3, -2, -1, 0, +1, +2, +3) Function: Determines wich of the orbitals of the same type the electron is in.