Electron Arrangements Electron Arrangements Energy Level also called




- Slides: 19

Electron Arrangements

Electron Arrangements Energy Level (also called orbit, shell, or orbital): where an electron can be found, at a specific distance outside the nucleus

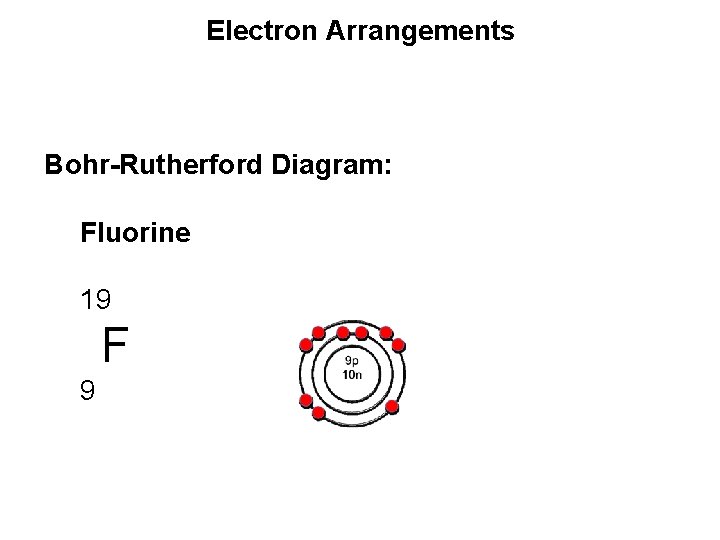
Electron Arrangements Bohr-Rutherford Diagram: Fluorine 19 F 9

Electron Arrangements. . . but we're mostly interested in the outermost shell

Electron Arrangements. . . but we're mostly interested in the outermost shell Valence shell: the outermost energy level, responsible for how the atom bonds with other atoms

Electron Arrangements. . . but we're mostly interested in the outermost shell Valence shell: the outermost energy level, responsible for how the atom bonds with other atoms Family (also called group): indicates the number of electrons in the valence shell e. g. Family IIIA has 3 valence electrons

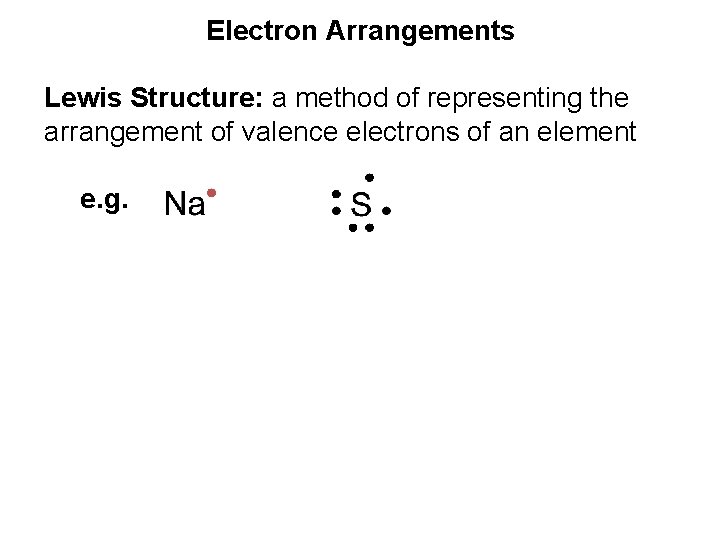
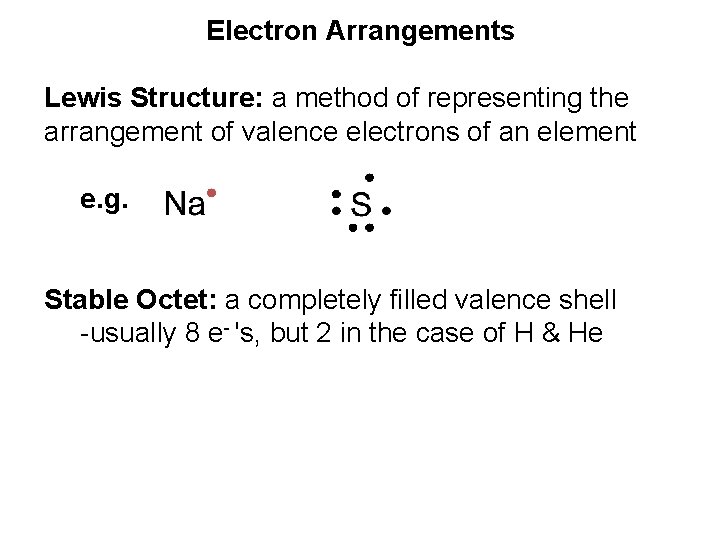
Electron Arrangements Lewis Structure: a method of representing the arrangement of valence electrons of an element e. g.

Electron Arrangements Lewis Structure: a method of representing the arrangement of valence electrons of an element e. g. Stable Octet: a completely filled valence shell -usually 8 e- 's, but 2 in the case of H & He

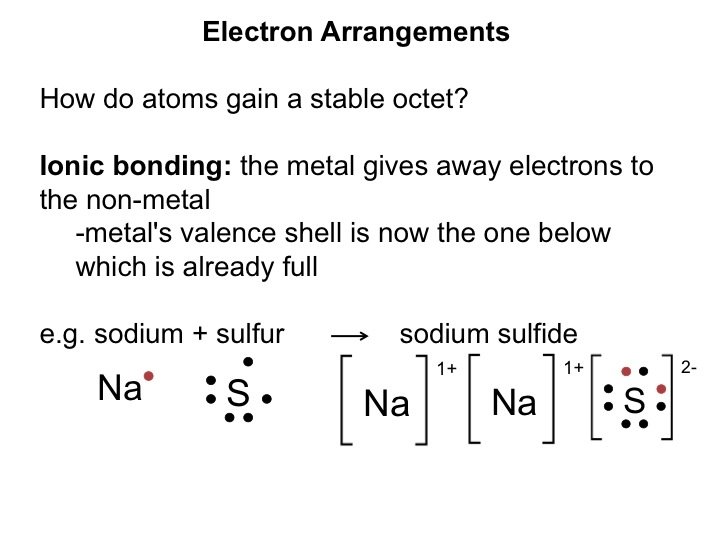
Electron Arrangements How do atoms gain a stable octet?

Electron Arrangements How do atoms gain a stable octet? Ionic bonding: the metal gives away electrons to the non-metal's valence shell is now the one below which is already full


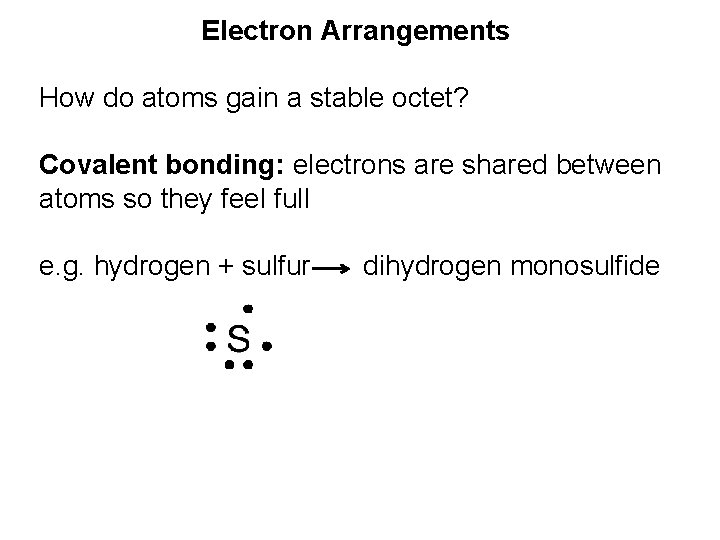
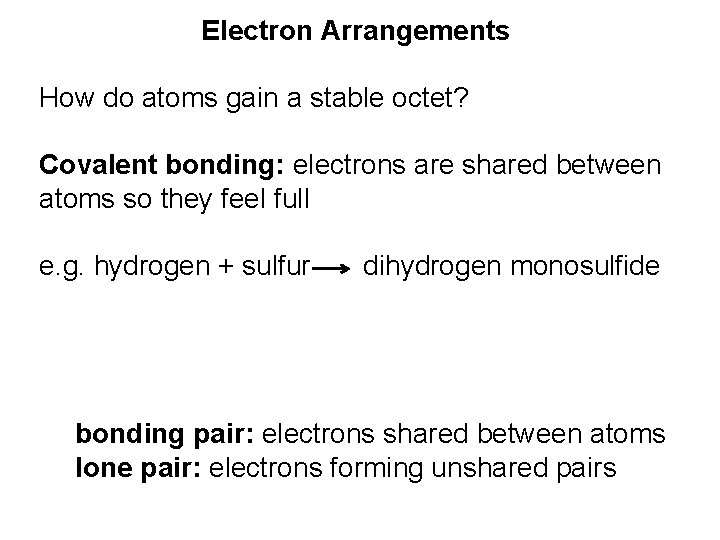
Electron Arrangements How do atoms gain a stable octet? Covalent bonding: electrons are shared between atoms so they feel full

Electron Arrangements How do atoms gain a stable octet? Covalent bonding: electrons are shared between atoms so they feel full e. g. hydrogen + sulfur dihydrogen monosulfide

Electron Arrangements How do atoms gain a stable octet? Covalent bonding: electrons are shared between atoms so they feel full e. g. hydrogen + sulfur dihydrogen monosulfide bonding pair: electrons shared between atoms lone pair: electrons forming unshared pairs

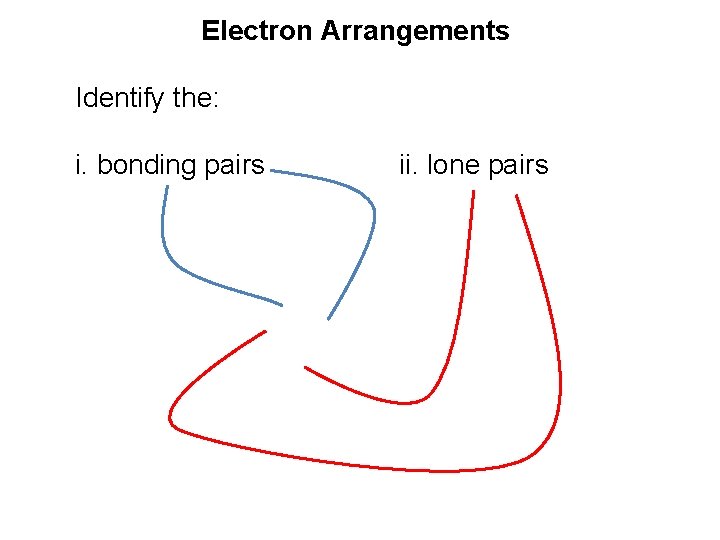
Electron Arrangements Identify the: i. bonding pairs ii. lone pairs

Electron Arrangements Identify the: i. bonding pairs ii. lone pairs

Electron Arrangements How do atoms gain a stable octet? Covalent bonding: electrons are shared between atoms so they feel full double bonds e. g. carbon + sulfur carbon disulfide

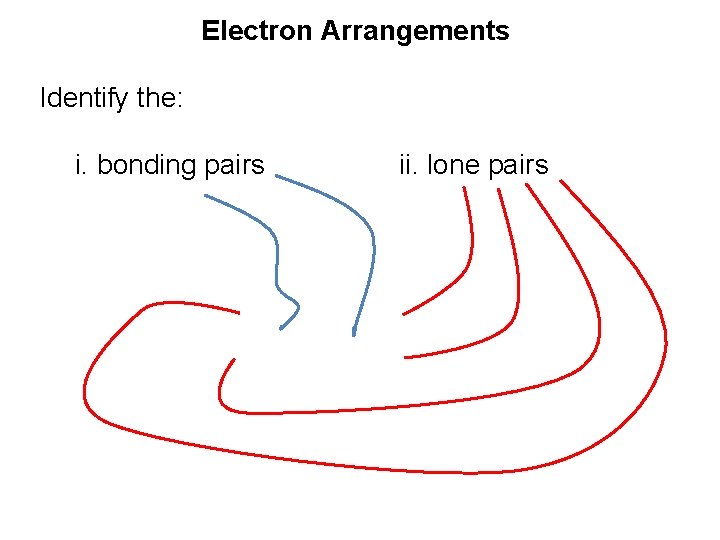
Electron Arrangements Identify the: i. bonding pairs ii. lone pairs

Electron Arrangements Identify the: i. bonding pairs ii. lone pairs