Electron Arrangement Planetary Model l The planetary model




- Slides: 32

Electron Arrangement

Planetary Model l The planetary model was developed by Bohr. Using this model, the sun represents the nucleus and the planets represent the electrons.

Electron Cloud Model l In this model, a cloud is used to represent the area surrounding the nucleus in which the electrons are located.

Heisenberg l Heisenberg stated that it is impossible to know both the location and the momentum of an electron at the same time. l This is commonly referred to as the Heisenberg Uncertainty Principle.

Schroedinger developed a mathematical equation. l The solution to the equation was a set of four quantum numbers for each electron in an atom. l This set of numbers was used to describe the area in the electron cloud in which the electron has the greatest probability of being located. l

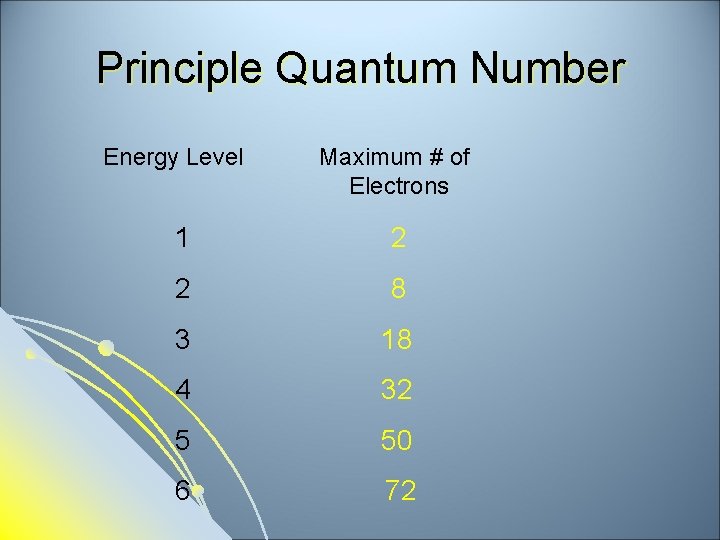
Quantum Numbers Principle Quantum Number (n)-indicates the size of the electron cloud and is commonly referred to as the energy level. l Each energy level has a maximum number of electrons. (2 n 2) l

Principle Quantum Number Energy Level Maximum # of Electrons 1 2 2 8 3 18 4 32 5 50 6 72

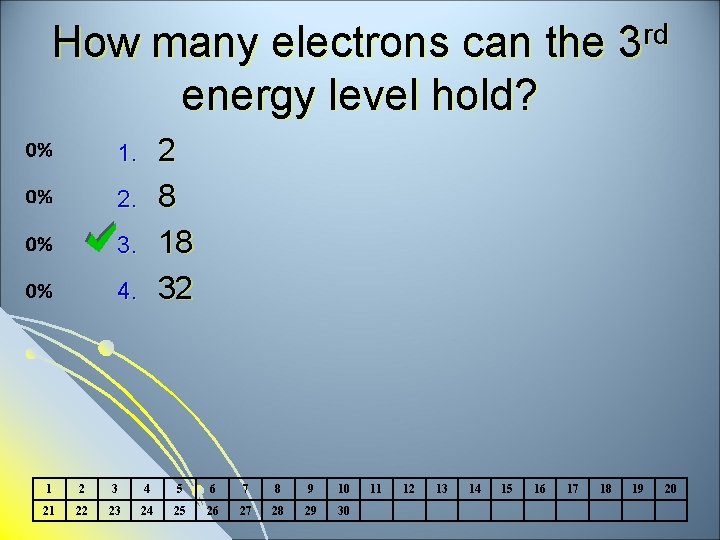
How many electrons can the energy level hold? rd 3 2 8 18 32 1. 2. 3. 4. 1 2 3 4 5 6 7 8 9 10 21 22 23 24 25 26 27 28 29 30 11 12 13 14 15 16 17 18 19 20

How many electrons can the level hold? th 7 32 49 98 125 1. 2. 3. 4. 10 1 2 3 4 5 6 7 8 9 10 21 22 23 24 25 26 27 28 29 30 11 12 13 14 15 16 17 18 19 20

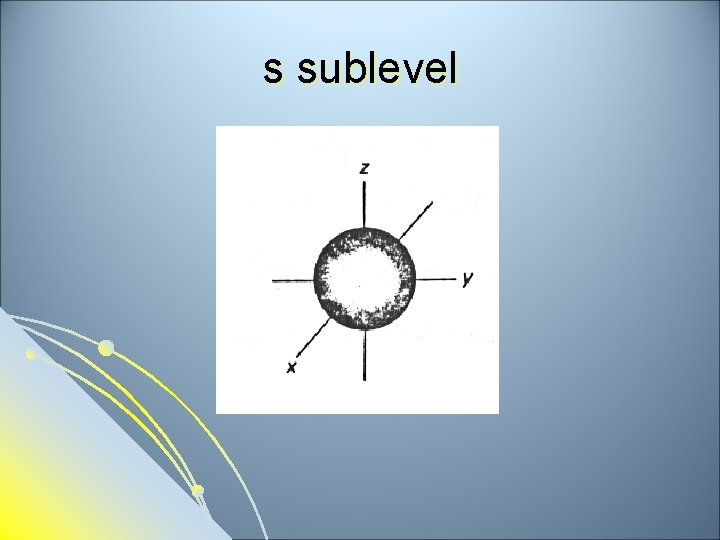
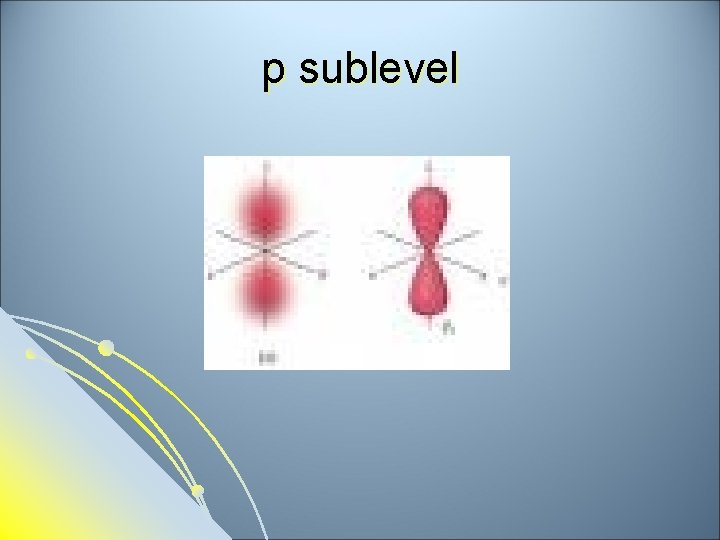
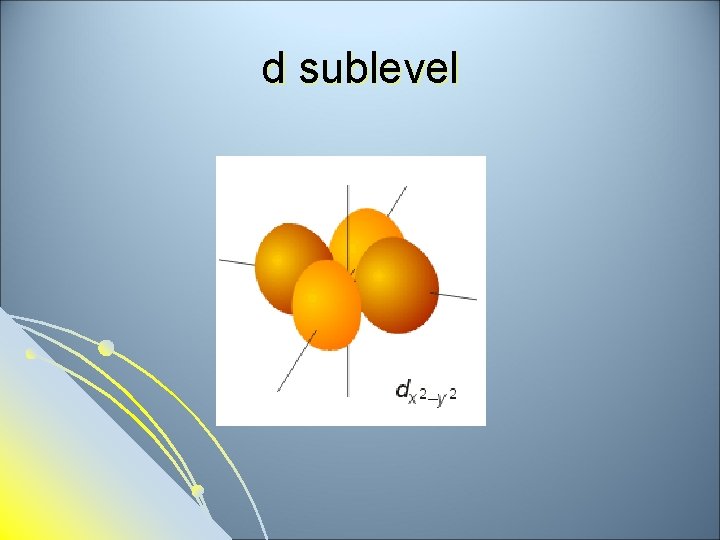
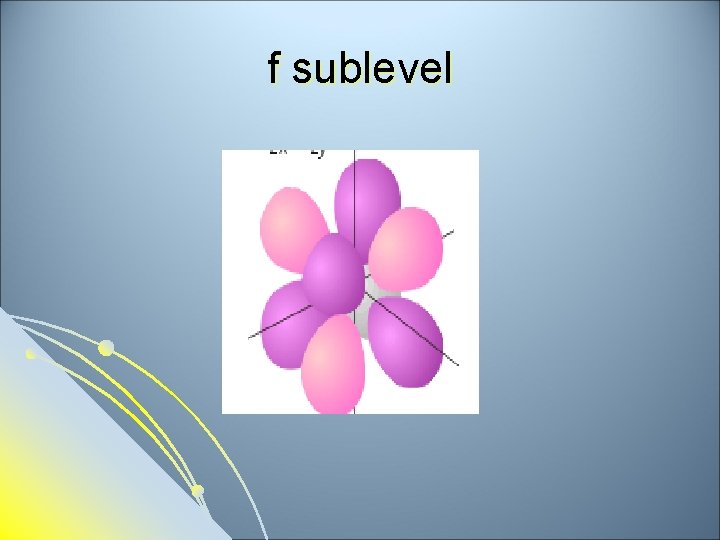
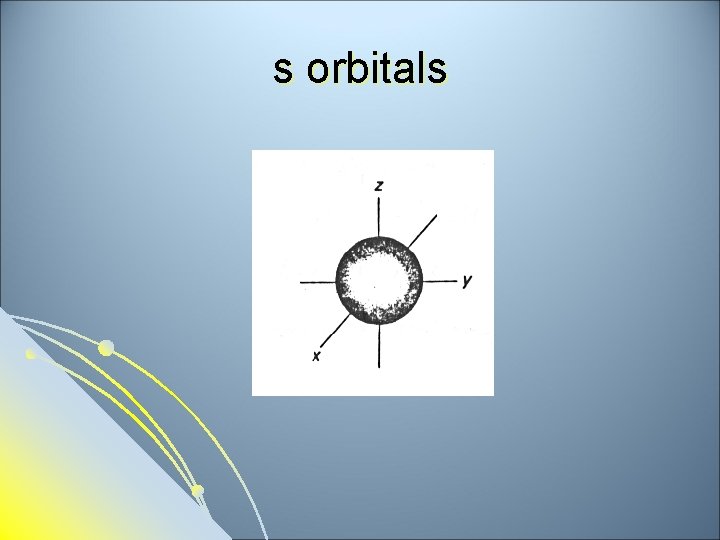
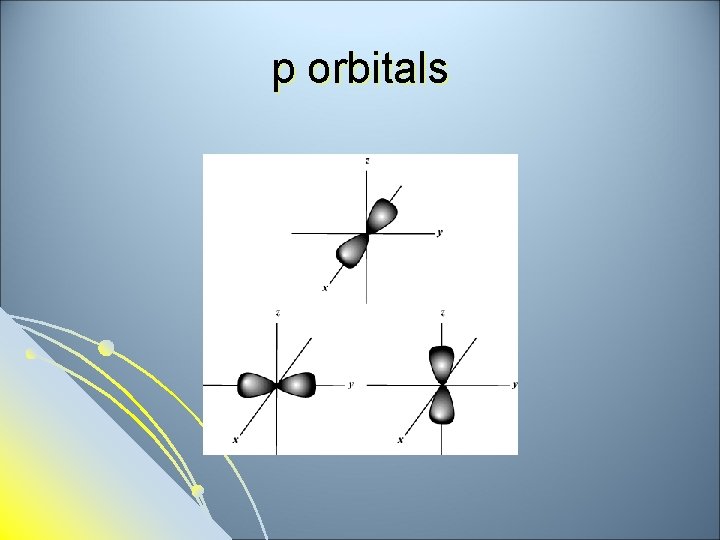
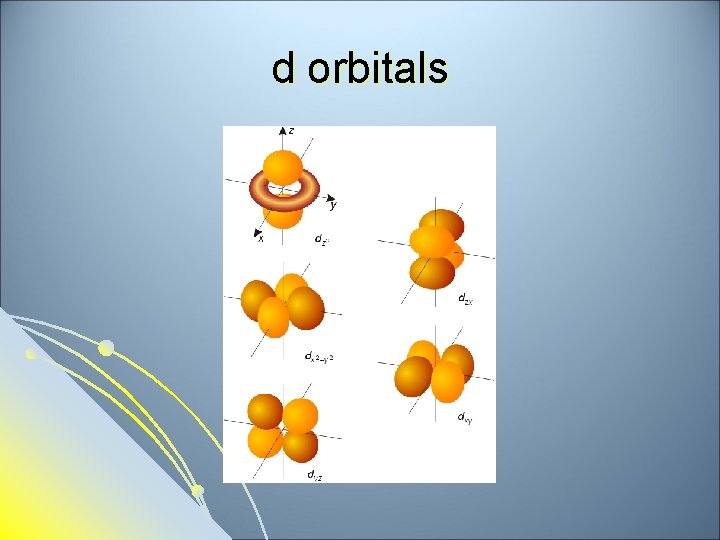
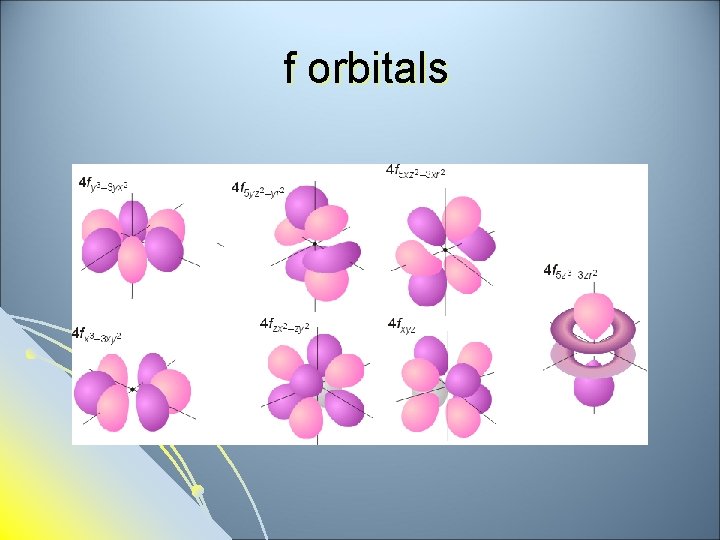
Subsidiary Quantum Number l l l Subsidiary Quantum Number (l)- indicates the shape of the electron cloud and is commonly referred to as the sublevel. Sublevels are represented by the letters s, p, d, and f. The s sublevel is spherical, the p sublevel is shaped like a dumbbell (2 lobes), the d sublevel has 4 lobes, and the f sublevel has 8 lobes. Each sublevel can hold a maximum number of electrons. Each energy level is made up of a maximum number of sublevels.

s sublevel

p sublevel

d sublevel

f sublevel

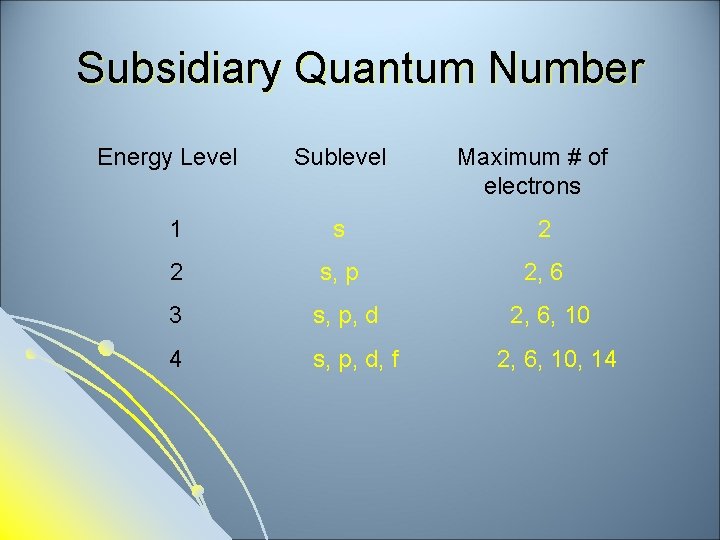
Subsidiary Quantum Number Energy Level Sublevel Maximum # of electrons 1 s 2 2 s, p 2, 6 3 s, p, d 2, 6, 10 4 s, p, d, f 2, 6, 10, 14

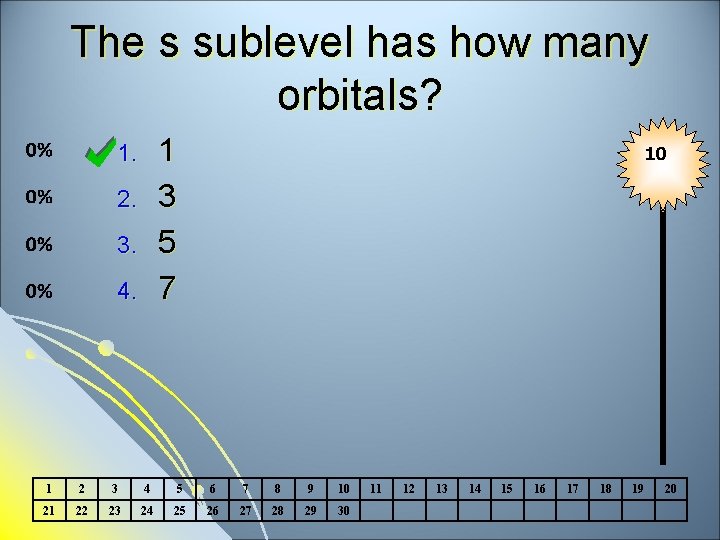
The p sublevel is what shape? Spherical Dumbbell 2 lobed 4 lobed 1. 2. 3. 4. 10 1 2 3 4 5 6 7 8 9 10 21 22 23 24 25 26 27 28 29 30 11 12 13 14 15 16 17 18 19 20

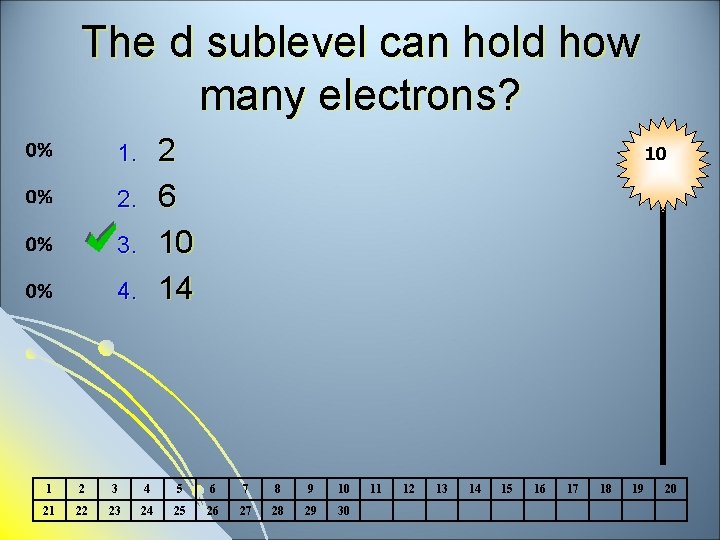
The d sublevel can hold how many electrons? 2 6 10 14 1. 2. 3. 4. 10 1 2 3 4 5 6 7 8 9 10 21 22 23 24 25 26 27 28 29 30 11 12 13 14 15 16 17 18 19 20

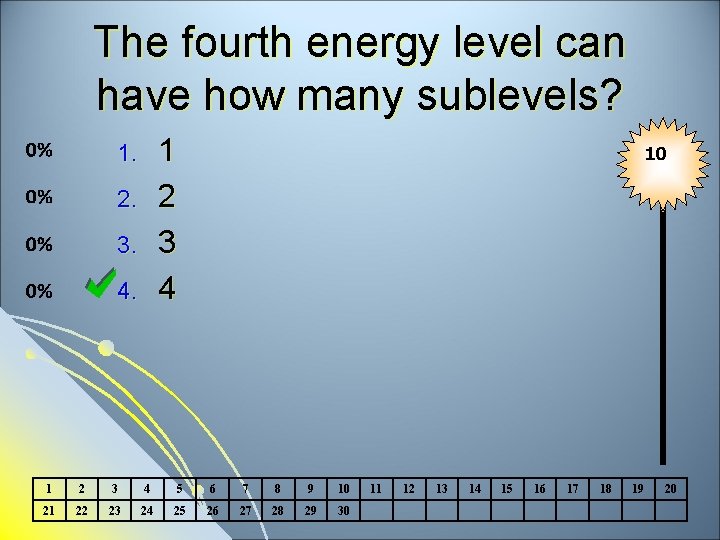
The fourth energy level can have how many sublevels? 1 2 3 4 1. 2. 3. 4. 10 1 2 3 4 5 6 7 8 9 10 21 22 23 24 25 26 27 28 29 30 11 12 13 14 15 16 17 18 19 20

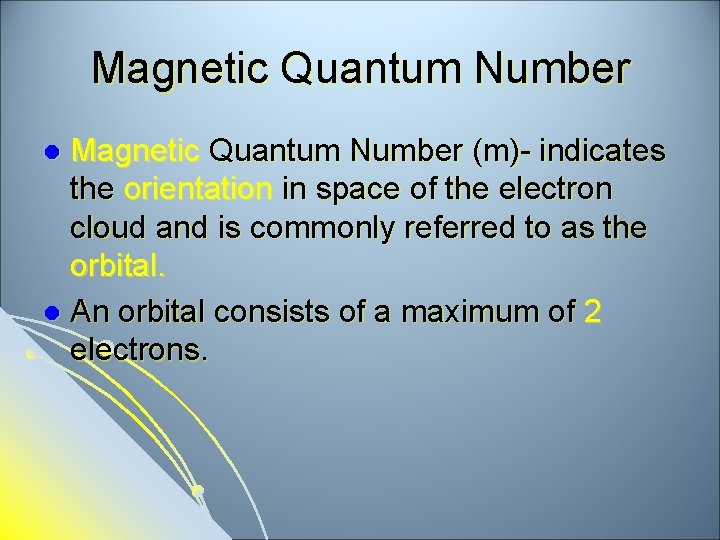
Magnetic Quantum Number (m)- indicates the orientation in space of the electron cloud and is commonly referred to as the orbital. l An orbital consists of a maximum of 2 electrons. l

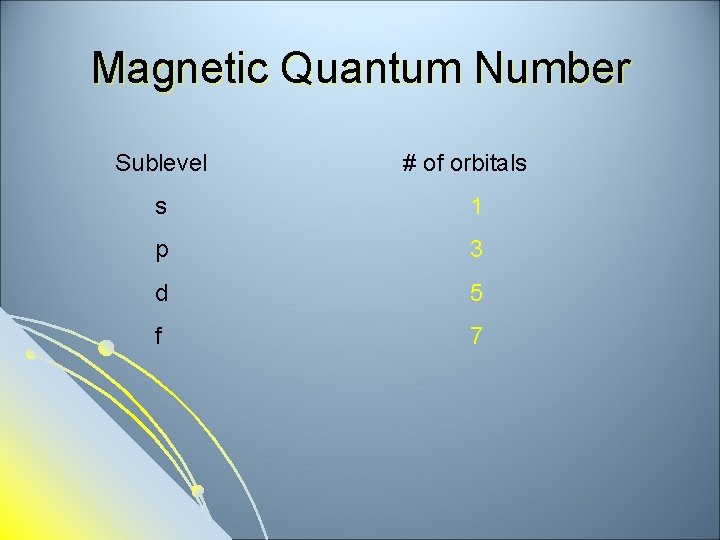
Magnetic Quantum Number Sublevel # of orbitals s 1 p 3 d 5 f 7

s orbitals

p orbitals

d orbitals

f orbitals

The s sublevel has how many orbitals? 1 3 5 7 1. 2. 3. 4. 10 1 2 3 4 5 6 7 8 9 10 21 22 23 24 25 26 27 28 29 30 11 12 13 14 15 16 17 18 19 20

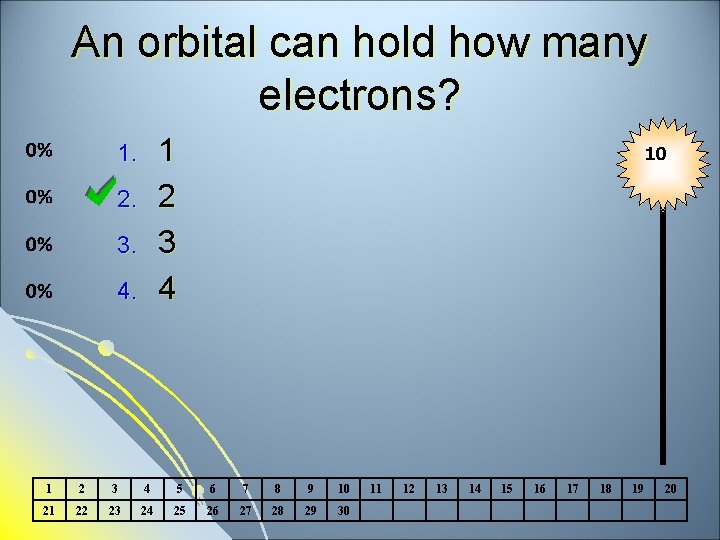
An orbital can hold how many electrons? 1 2 3 4 1. 2. 3. 4. 10 1 2 3 4 5 6 7 8 9 10 21 22 23 24 25 26 27 28 29 30 11 12 13 14 15 16 17 18 19 20

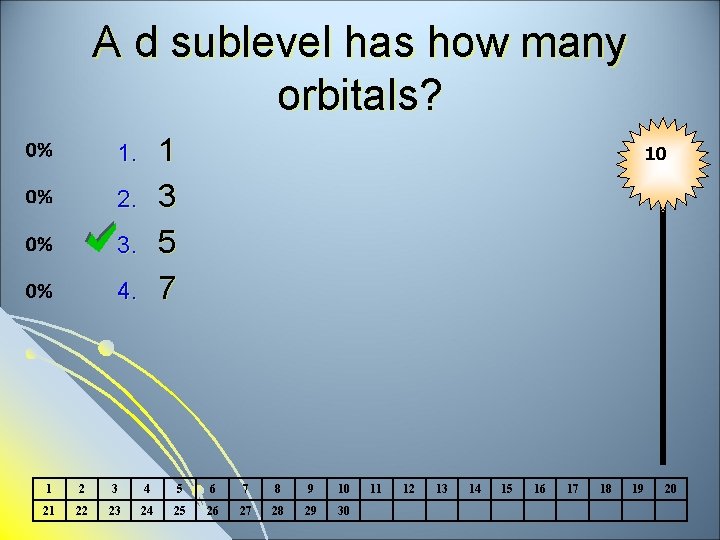
A d sublevel has how many orbitals? 1 3 5 7 1. 2. 3. 4. 10 1 2 3 4 5 6 7 8 9 10 21 22 23 24 25 26 27 28 29 30 11 12 13 14 15 16 17 18 19 20

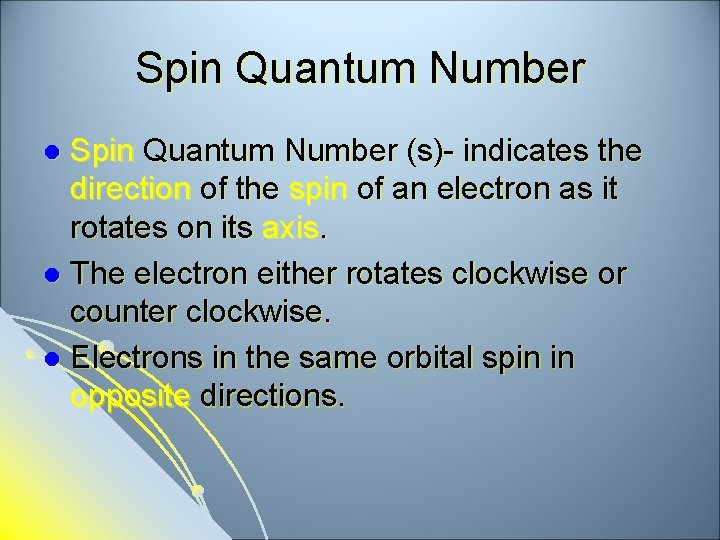
Spin Quantum Number (s)- indicates the direction of the spin of an electron as it rotates on its axis. l The electron either rotates clockwise or counter clockwise. l Electrons in the same orbital spin in opposite directions. l

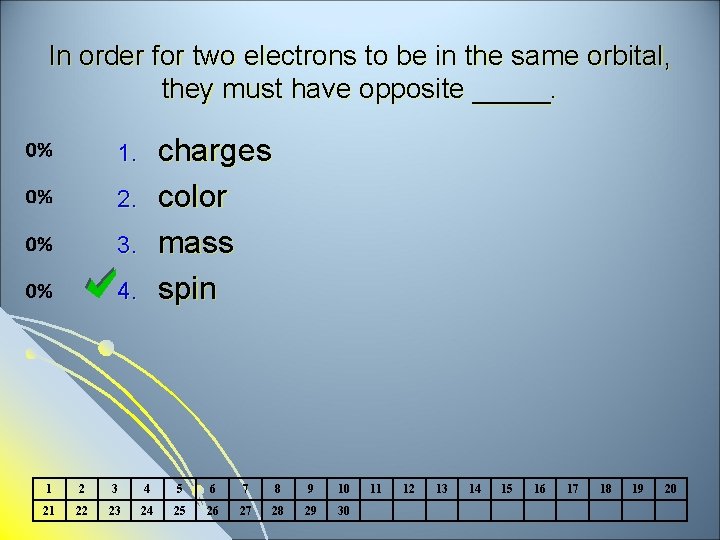
In order for two electrons to be in the same orbital, they must have opposite _____. charges color mass spin 1. 2. 3. 4. 1 2 3 4 5 6 7 8 9 10 21 22 23 24 25 26 27 28 29 30 11 12 13 14 15 16 17 18 19 20

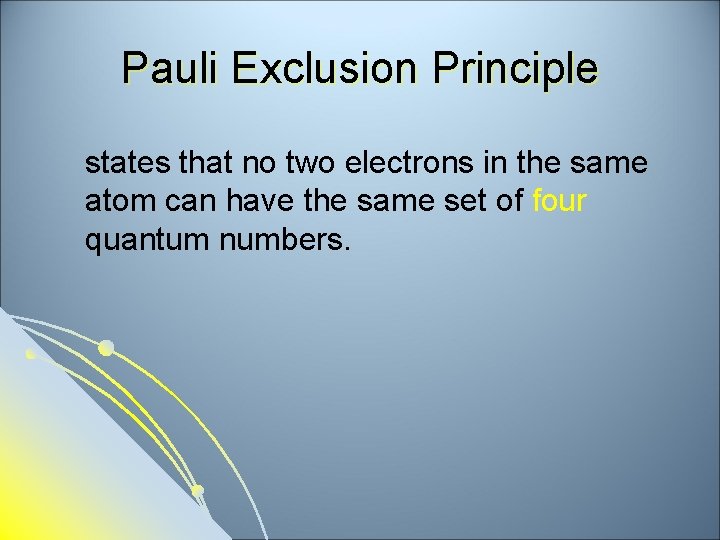
Pauli Exclusion Principle states that no two electrons in the same atom can have the same set of four quantum numbers.

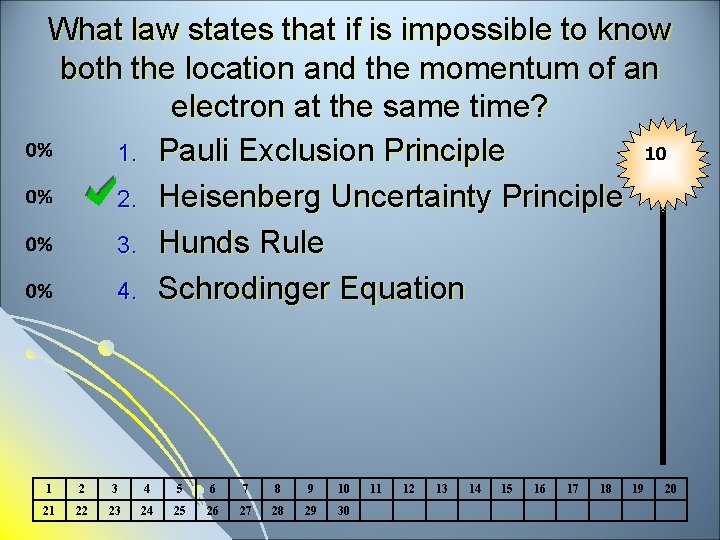
What law states that if is impossible to know both the location and the momentum of an electron at the same time? 10 1. Pauli Exclusion Principle 2. Heisenberg Uncertainty Principle 3. Hunds Rule 4. Schrodinger Equation 1 2 3 4 5 6 7 8 9 10 21 22 23 24 25 26 27 28 29 30 11 12 13 14 15 16 17 18 19 20

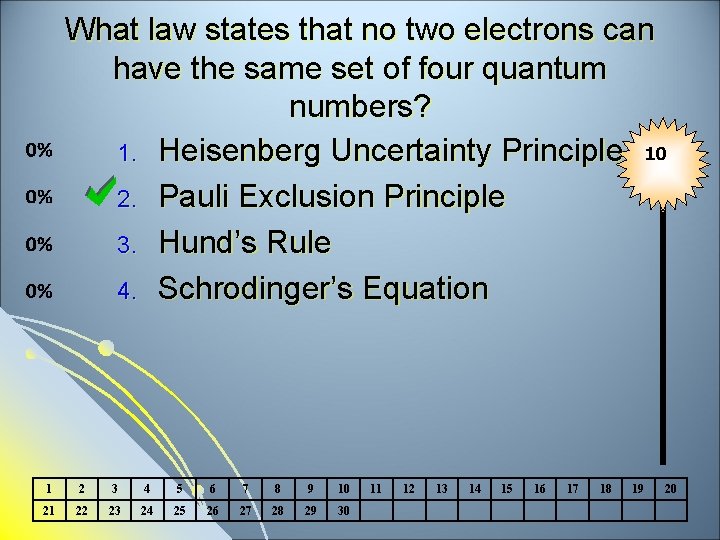
What law states that no two electrons can have the same set of four quantum numbers? 1. Heisenberg Uncertainty Principle 10 2. Pauli Exclusion Principle 3. Hund’s Rule 4. Schrodinger’s Equation 1 2 3 4 5 6 7 8 9 10 21 22 23 24 25 26 27 28 29 30 11 12 13 14 15 16 17 18 19 20