Electron Arrangement and Quantum Numbers Quantum Numbers According

Electron Arrangement and Quantum Numbers

Quantum Numbers According to the QM Model, each electron in an atom can be described by 4 quantum numbers QUANTUM ORGANIZATION • Energy Level (shell) • Sublevel (subshell) • Atomic Orbital • Spin
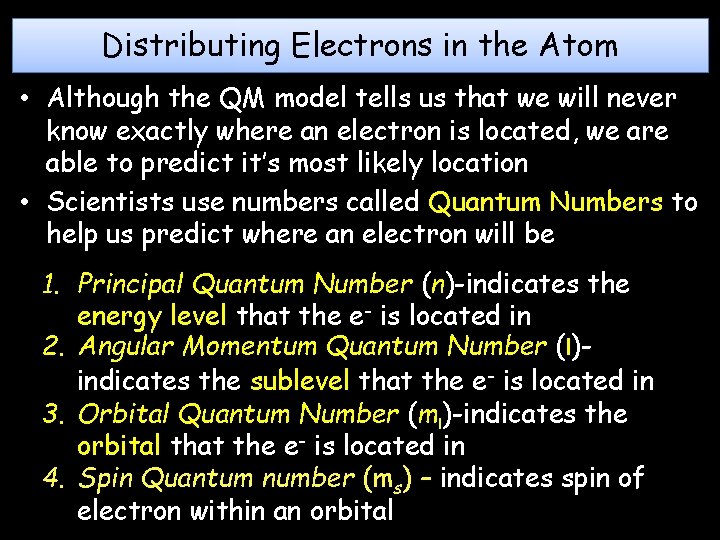
Distributing Electrons in the Atom • Although the QM model tells us that we will never know exactly where an electron is located, we are able to predict it’s most likely location • Scientists use numbers called Quantum Numbers to help us predict where an electron will be 1. Principal Quantum Number (n)-indicates the energy level that the e- is located in 2. Angular Momentum Quantum Number (l)indicates the sublevel that the e- is located in 3. Orbital Quantum Number (ml)-indicates the orbital that the e- is located in 4. Spin Quantum number (ms) – indicates spin of electron within an orbital

Principal QN • Also known as the Energy Level QN • Symbolized by “n”, it denotes the energy level (shell) in which an electron is located • Maximum number of electrons in an energy level = 2 n 2 • The energy of an electron in an atom depends on n: The larger the value of n, the higher the energy of the e-
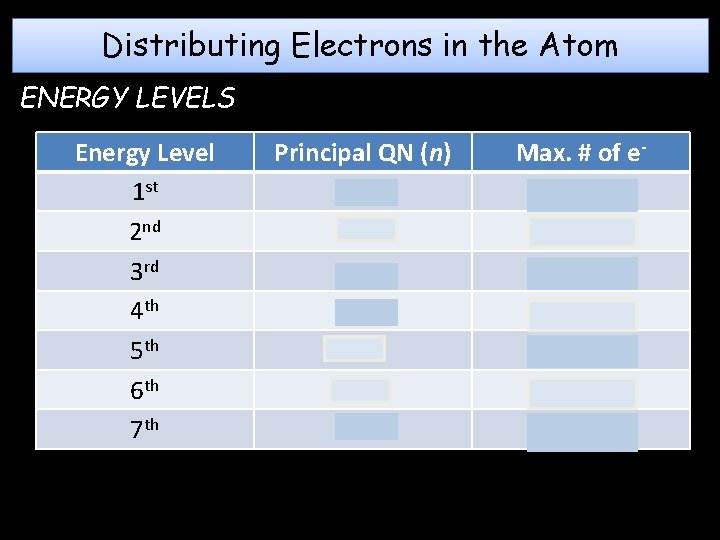
Distributing Electrons in the Atom ENERGY LEVELS Energy Level 1 st 2 nd 3 rd 4 th 5 th 6 th 7 th Principal QN (n) 1 2 3 4 5 6 7 Max. # of e 2(1)2 = 2 2(2)2 = 8 2(3)2 = 18 2(4)2 = 32 2(5)2 = 50 2(6)2 = 72 2(7)2 = 98
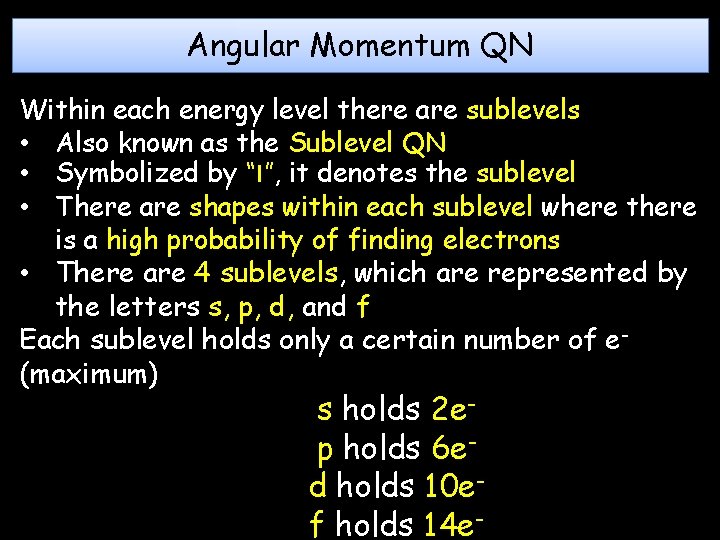
Angular Momentum QN Within each energy level there are sublevels • Also known as the Sublevel QN • Symbolized by “l”, it denotes the sublevel • There are shapes within each sublevel where there is a high probability of finding electrons • There are 4 sublevels, which are represented by the letters s, p, d, and f Each sublevel holds only a certain number of e(maximum) s holds 2 ep holds 6 ed holds 10 ef holds 14 e-
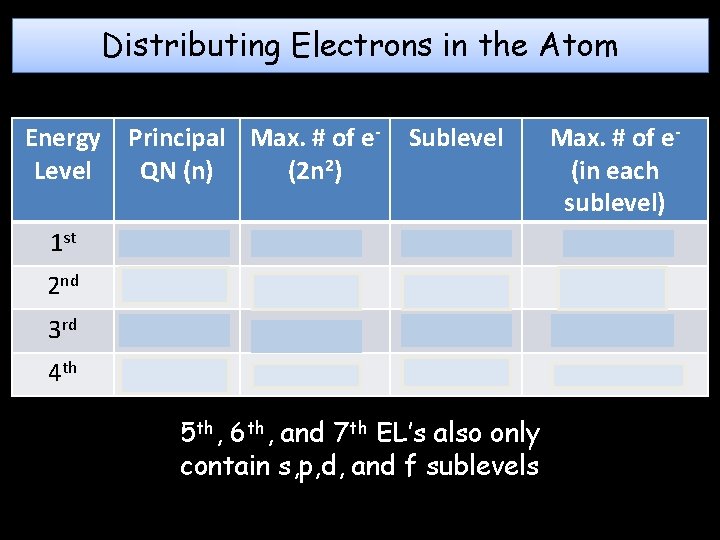
Distributing Electrons in the Atom Energy Level Principal Max. # of e. QN (n) (2 n 2) Sublevel 1 st 1 2 s Max. # of e(in each sublevel) 2 2 nd 2 8 s, p 2, 6 3 rd 3 18 s, p, d 2, 6, 10 4 th 4 32 s, p, d, f 2, 6, 10, 14 5 th, 6 th, and 7 th EL’s also only contain s, p, d, and f sublevels
Magnetic Quantum QN ORBITALS - Each sublevel is made up of orbitals Orbital: 3 -D shape which describes the region where an electron will most likely be found Each orbital can hold a maximum of 2 electrons – Also known as the Orbital QN • Symbolized by “ml”, it denotes the electron’s orientation in space around the nucleus (specifies the orbital an electron is located in) Each sublevel contains a certain number of orbitals s has 1 orbital p has 3 orbitals d has 5 orbitals f has 7 orbitals • “e- cloud” is a combination of all orbitals in an atom •
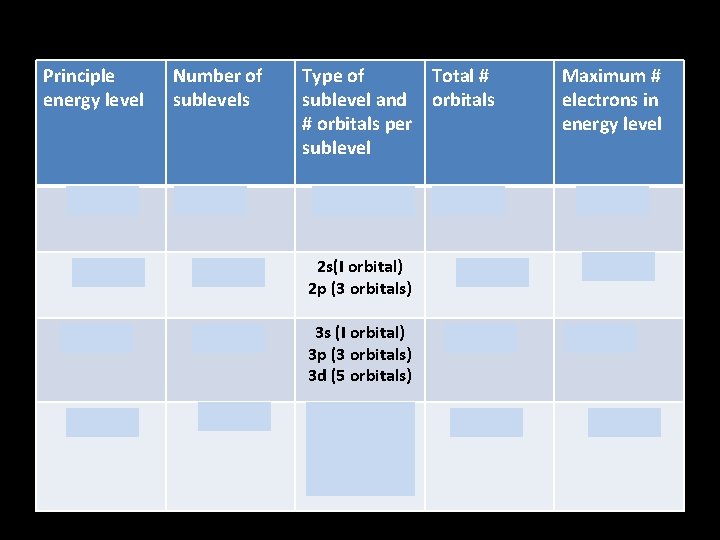
Principle energy level Number of sublevels Type of Total # sublevel and orbitals # orbitals per sublevel Maximum # electrons in energy level n=1 1 1 s(1 orbital) 1 2 n=2 2 2 s(I orbital) 2 p (3 orbitals) 4 8 n=3 3 3 s (I orbital) 3 p (3 orbitals) 3 d (5 orbitals) 9 18 n=4 4 4 s (I orbital) 4 p (3 orbitals) 4 d (5 orbitals) 4 f (7 orbitals) 16 32
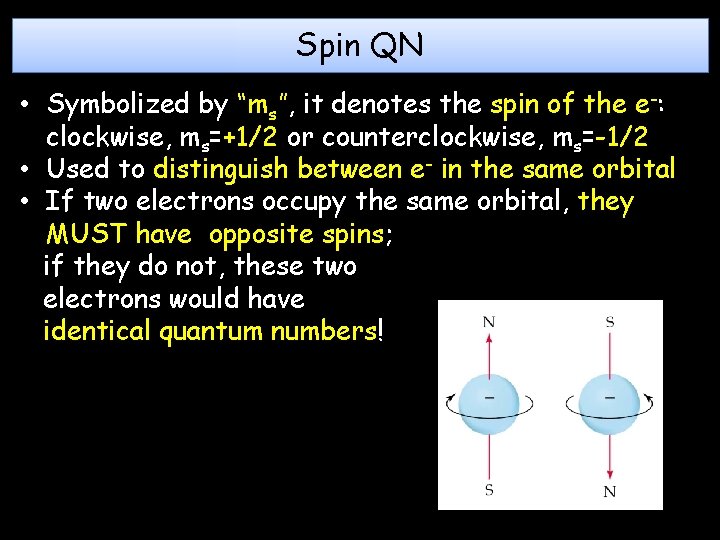
Spin QN • Symbolized by “ms”, it denotes the spin of the e-: clockwise, ms=+1/2 or counterclockwise, ms=-1/2 • Used to distinguish between e- in the same orbital • If two electrons occupy the same orbital, they MUST have opposite spins; if they do not, these two electrons would have identical quantum numbers!
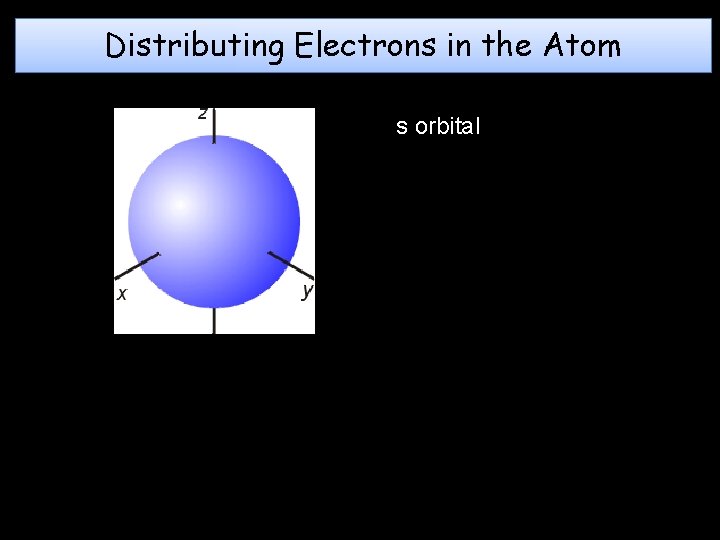
Distributing Electrons in the Atom s orbital
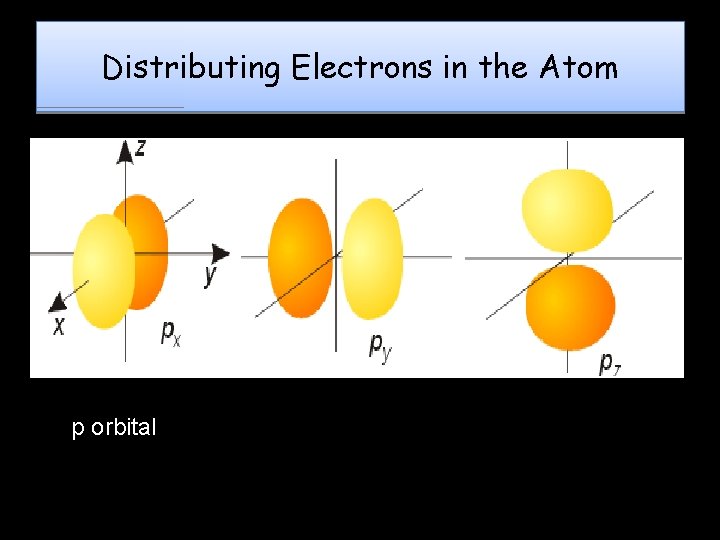
Distributing Electrons in the Atom p orbital
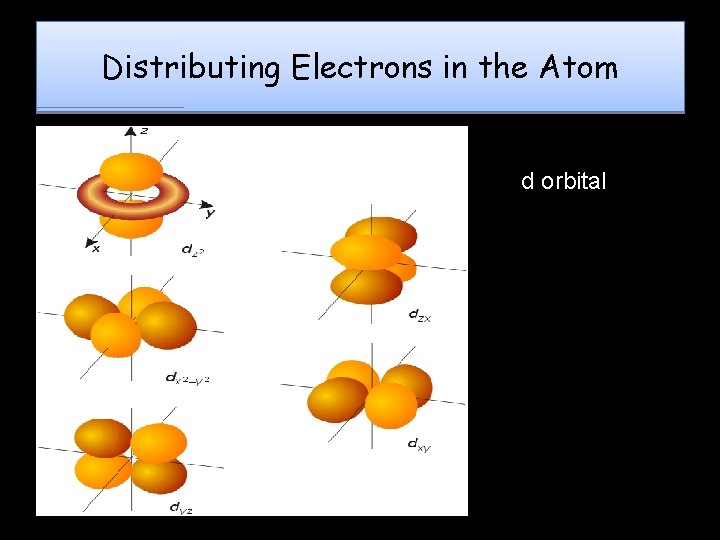
Distributing Electrons in the Atom d orbital
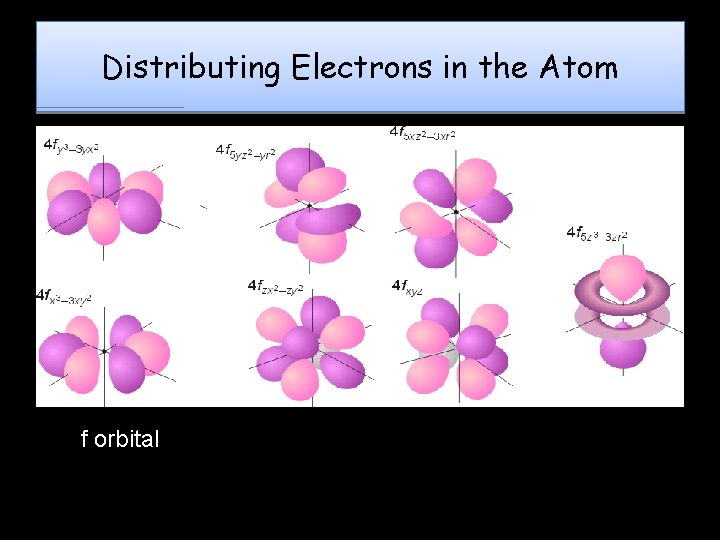
Distributing Electrons in the Atom f orbital
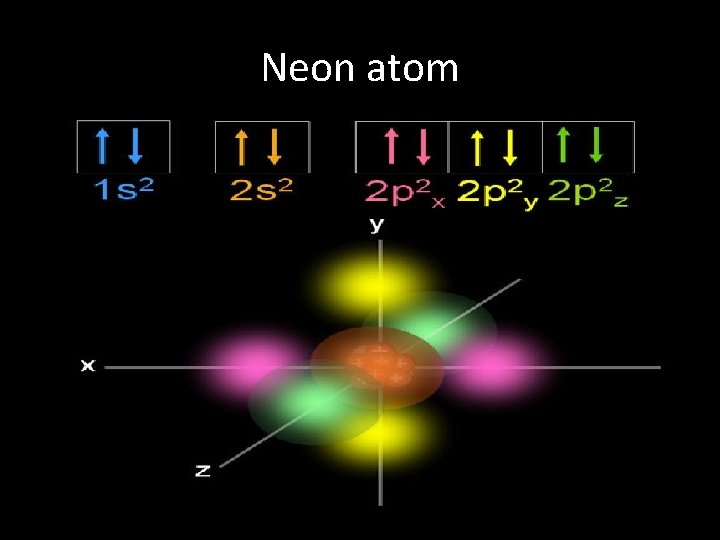
Neon atom
- Slides: 15