Electromagnetic Spectrum Calculations Using Plancks Constant Determining the


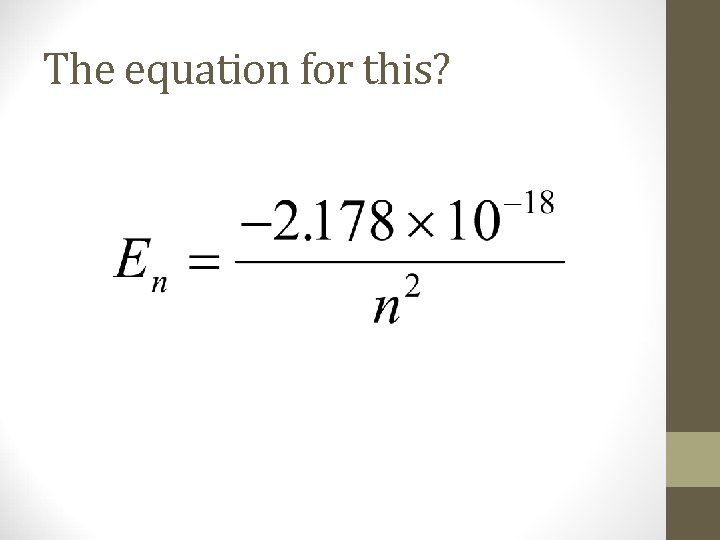
























- Slides: 45

Electromagnetic Spectrum Calculations Using Planck’s Constant…

Determining the Speed of Light • Galileo tried unsuccessfully to determine the speed of light using an assistant with a lantern on a distant hilltop.

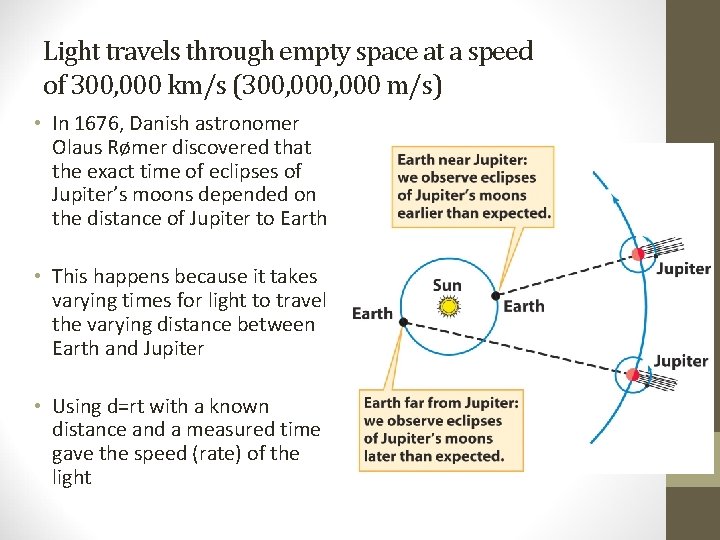
Light travels through empty space at a speed of 300, 000 km/s (300, 000 m/s) • In 1676, Danish astronomer Olaus Rømer discovered that the exact time of eclipses of Jupiter’s moons depended on the distance of Jupiter to Earth • This happens because it takes varying times for light to travel the varying distance between Earth and Jupiter • Using d=rt with a known distance and a measured time gave the speed (rate) of the light

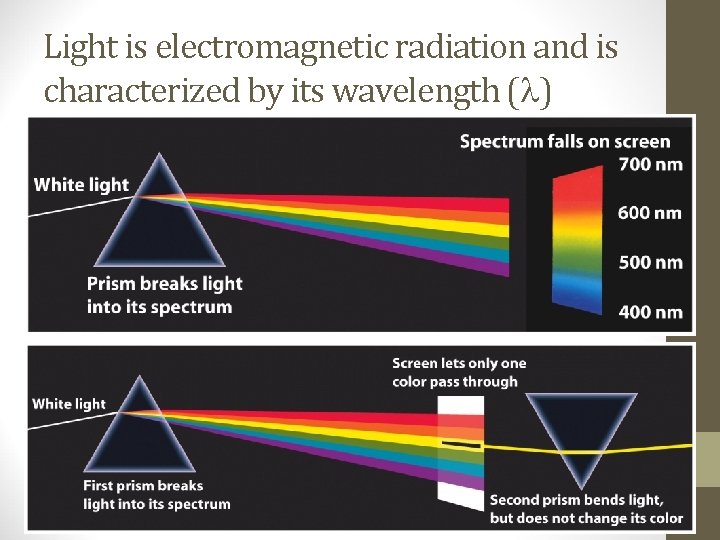
Light is electromagnetic radiation and is characterized by its wavelength ( )

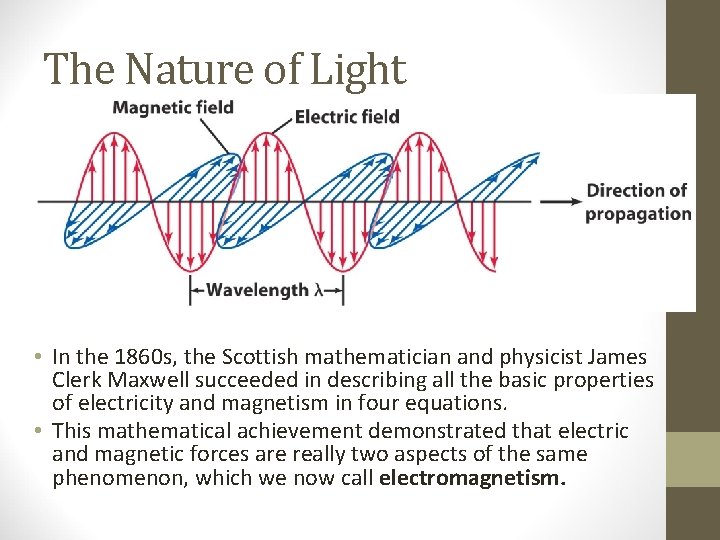
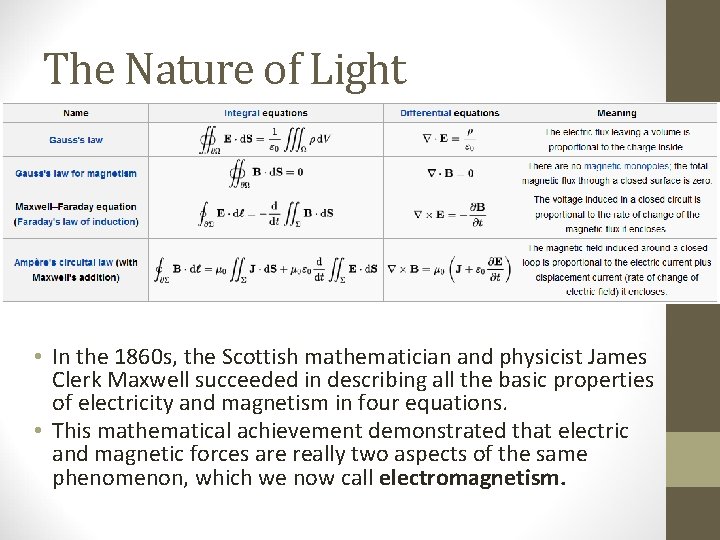
The Nature of Light • In the 1860 s, the Scottish mathematician and physicist James Clerk Maxwell succeeded in describing all the basic properties of electricity and magnetism in four equations. • This mathematical achievement demonstrated that electric and magnetic forces are really two aspects of the same phenomenon, which we now call electromagnetism.

The Nature of Light • First durable color photographic image, demonstrated by James Clerk Maxwell in an 1861 lecture. • (It’s a tartan.

The Nature of Light • In the 1860 s, the Scottish mathematician and physicist James Clerk Maxwell succeeded in describing all the basic properties of electricity and magnetism in four equations. • This mathematical achievement demonstrated that electric and magnetic forces are really two aspects of the same phenomenon, which we now call electromagnetism.

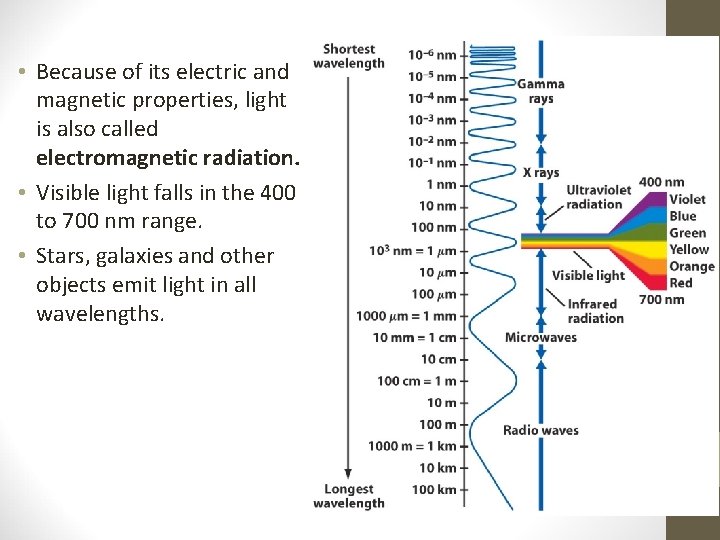
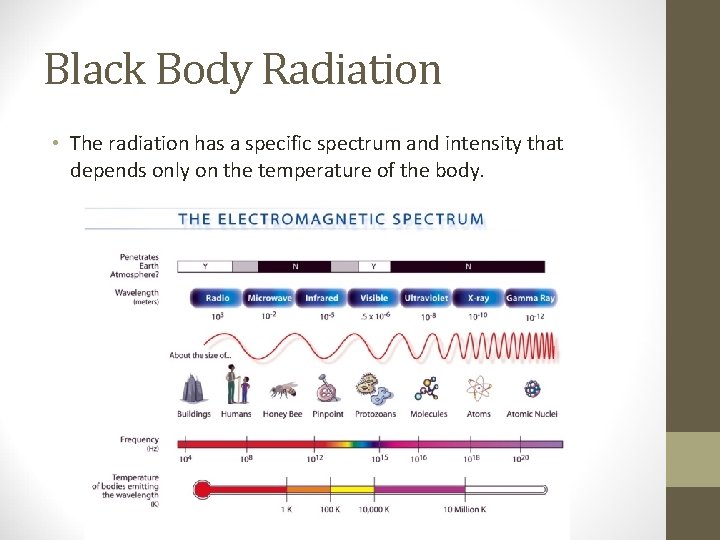
• Because of its electric and magnetic properties, light is also called electromagnetic radiation. • Visible light falls in the 400 to 700 nm range. • Stars, galaxies and other objects emit light in all wavelengths.

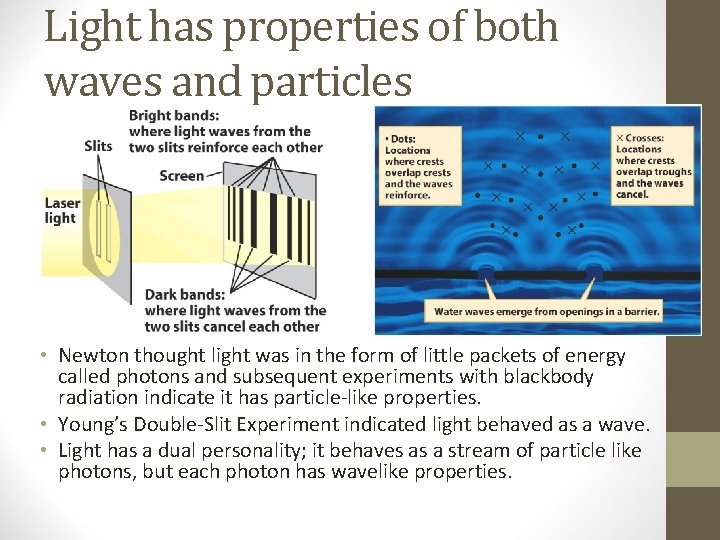
Light has properties of both waves and particles • Newton thought light was in the form of little packets of energy called photons and subsequent experiments with blackbody radiation indicate it has particle-like properties. • Young’s Double-Slit Experiment indicated light behaved as a wave. • Light has a dual personality; it behaves as a stream of particle like photons, but each photon has wavelike properties.

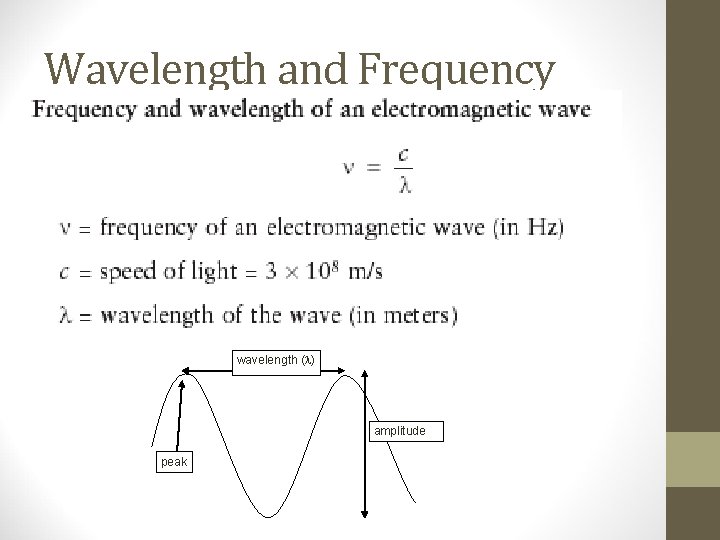
Wavelength and Frequency wavelength ( ) amplitude peak

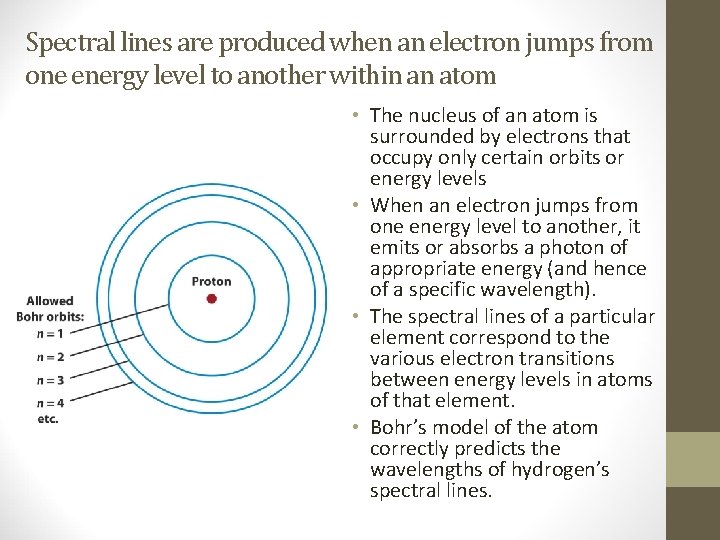
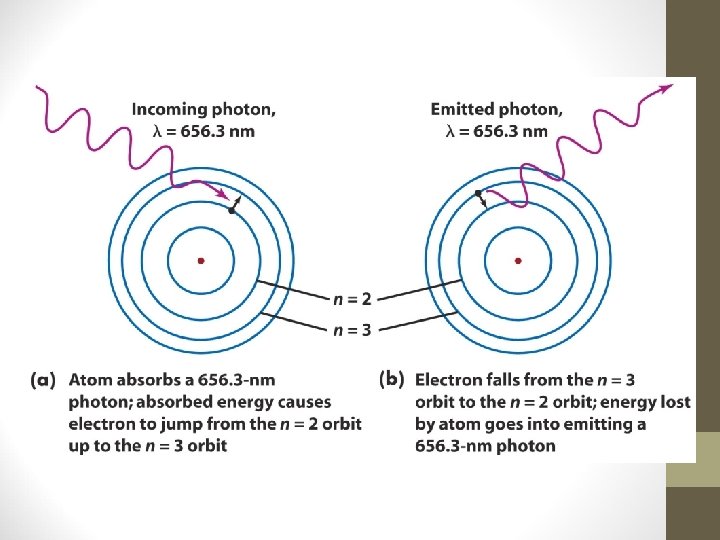
Spectral lines are produced when an electron jumps from one energy level to another within an atom • The nucleus of an atom is surrounded by electrons that occupy only certain orbits or energy levels • When an electron jumps from one energy level to another, it emits or absorbs a photon of appropriate energy (and hence of a specific wavelength). • The spectral lines of a particular element correspond to the various electron transitions between energy levels in atoms of that element. • Bohr’s model of the atom correctly predicts the wavelengths of hydrogen’s spectral lines.

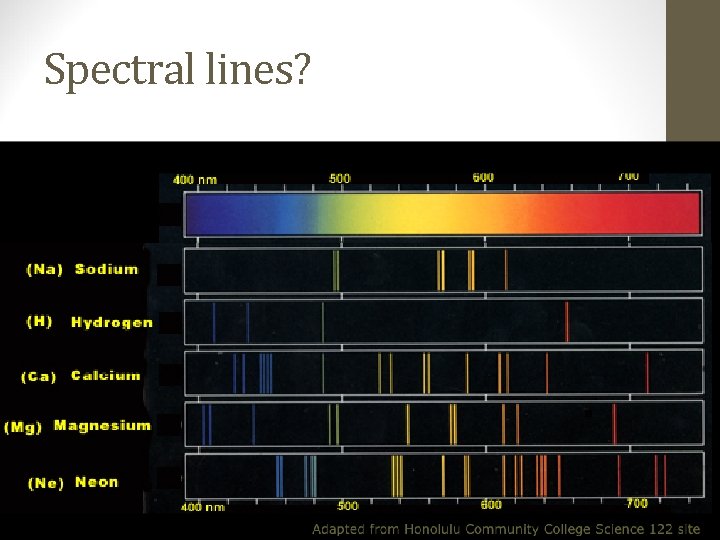
Spectral lines?
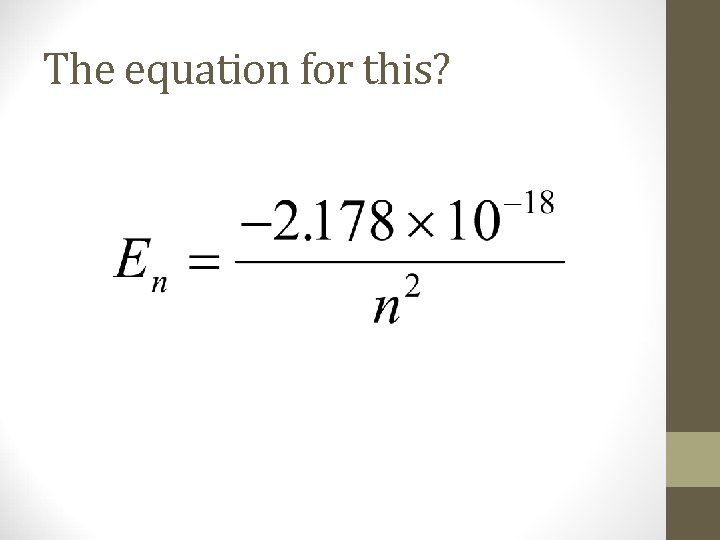
The equation for this?


Who was Max Planck? • In 1900, German physicist Max Planck , “the founder of the quantum theory”, (1858 -1947) was trying to model the broad smooth spectrum of electromagnetic radiation (i. e. , light) emitted by a warm body. • This “black body radiation” is what you see coming from the sun, the filament of an incandescent light bulb, or a hot electric stove element. • Its ‘spectrum’, the range of frequencies making up the radiation, is readily displayed by a prism or a diffraction grating. • In explaining the shape of the black body spectrum, Planck assumed that the electromagnetic radiation came not in continuous waves of energy, but in discrete clumps of energy which we now call photons. • Planck postulated the ‘photons’, at each frequency have a discrete. A energy. E = hf, where E is the energy of the photon in Joules, f is the frequency in Hertz, and h is Planck’s constant. Picture credit: http: //en. wikipedia. org/wiki/File: Max_Planck. png A. distinct, unique separate,

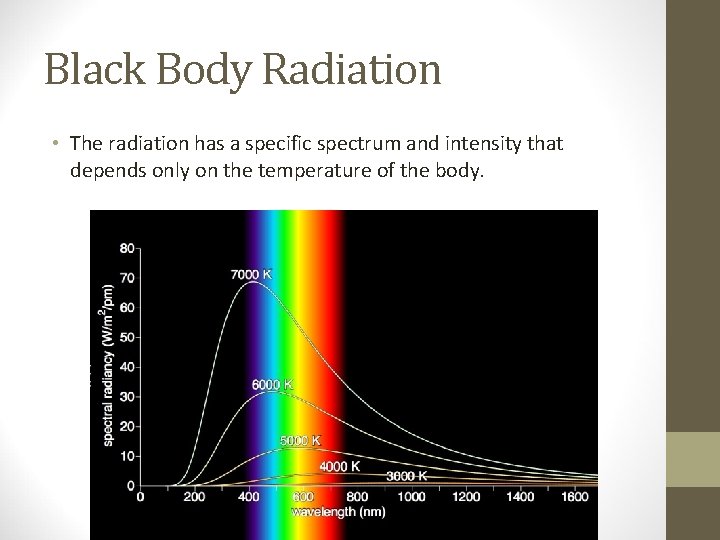
Black Body Radiation • The radiation has a specific spectrum and intensity that depends only on the temperature of the body.

Black Body Radiation • The radiation has a specific spectrum and intensity that depends only on the temperature of the body.

Who was Max Planck? • The existence of a smallest unit of light energy is one of the foundations of quantum mechanics. • The symbol (h) is used to denote Planck’s constant, which he discovered in 1899. It is used as a proportionality constant between the energy and frequency of an electromagnetic wave. (NOTE: We use it to describe the energy of a photon. ) • h = 6. 626 x 10 -34 Js (joule seconds) If you need more than this, go here: http: //web. mit. edu/lululiu/Public/pixx/not-pixx/photoelectric. pdf Picture credit: http: //en. wikipedia. org/wiki/File: Max_Planck. png

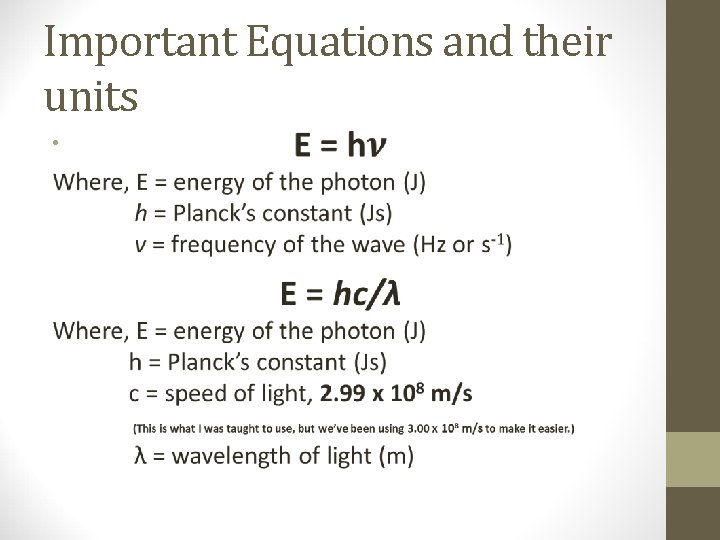
Important Equations and their units •

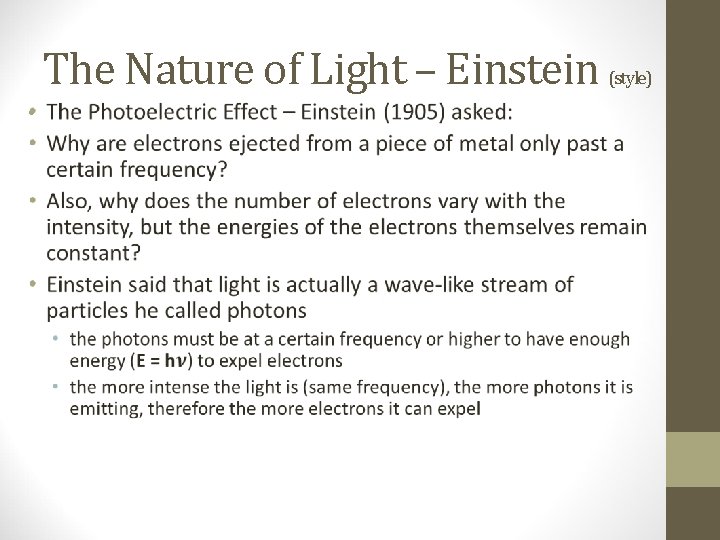
The Nature of Light – Einstein • (style)

Planck’s Practice Problems 1. When we see light from a neon sign, we are observing radiation from excited neon atoms. If this radiation has a wavelength of 640 nm, what is the energy of the photon being emitted?

Planck’s Practice Problems 1. When we see light from a neon sign, we are observing radiation from excited neon atoms. If this radiation has a wavelength of 640 nm, what is the energy of the photon being emitted? A. Pick your equation!

Planck’s Practice Problems 1. When we see light from a neon sign, we are observing radiation from excited neon atoms. If this radiation has a wavelength of 640 nm, what is the energy of the photon being emitted? A. Pick your equation! B. E=hν or E=hc/λ

Planck’s Practice Problems 1. When we see light from a neon sign, we are observing radiation from excited neon atoms. If this radiation has a wavelength of 640 nm, what is the energy of the photon being emitted? A. Pick your equation! B. E=hν or E=hc/λ You know me, I like this one: E=hc/λ

Planck’s Practice Problems 1. When we see light from a neon sign, we are observing radiation from excited neon atoms. If this radiation has a wavelength of 640 nm, what is the energy of the photon being emitted? A. Pick your equation! B. E=hν or E=hc/λ You know me, I like this one: E=hc/λ C. E=(6. 626× 10 -34 Js)(3. 00× 108 m/s) / 640 x 10 -9 m (or you can use: 6. 40 x 10 -7 m)

Planck’s Practice Problems 1. When we see light from a neon sign, we are observing radiation from excited neon atoms. If this radiation has a wavelength of 640 nm, what is the energy of the photon being emitted? A. Pick your equation! B. E=hν or E=hc/λ You know me, I like this one: E=hc/λ C. E=(6. 626× 10 -34 Js)(3. 00× 108 m/s) / 640 x 10 -9 m (or you can use: 6. 40 x 10 -7 m) E= -19 3. 11 x 10 J

Planck’s Practice Problems 2. Light with a wavelength of 614. 5 nm looks orange. What is the energy, in joules, of a photon of this orange light? A. Pick your equation!

Planck’s Practice Problems 2. Light with a wavelength of 614. 5 nm looks orange. What is the energy, in joules, of a photon of this orange light? A. Pick your equation! B. E=hν or E=hc/λ You know me, I like this one: E=hc/λ

Planck’s Practice Problems 2. Light with a wavelength of 614. 5 nm looks orange. What is the energy, in joules, of a photon of this orange light? A. Pick your equation! B. E=hν or E=hc/λ You know me, I like this one: E=hc/λ C. E=(6. 626× 10 -34 Js)(3. 00× 108 m/s) / 614. 5 x 10 -9 m (or you can use: 6. 145 x 10 -7 m)

Planck’s Practice Problems 2. Light with a wavelength of 614. 5 nm looks orange. What is the energy, in joules, of a photon of this orange light? A. Pick your equation! B. E=hν or E=hc/λ You know me, I like this one: E=hc/λ C. E=(6. 626× 10 -34 Js)(3. 00× 108 m/s) / 614. 5 x 10 -9 m (or you can use: 6. 145 x 10 -7 m) E= -19 3. 23 x 10 J

Planck’s Practice Problems 3. A photon of light produced by a surgical laser has an energy of 3. 027 x 10 -19 J. Calculate the frequency and the wavelength of the photon. A. Pick your equation!

Planck’s Practice Problems 3. A photon of light produced by a surgical laser has an energy of 3. 027 x 10 -19 J. Calculate the frequency and the wavelength of the photon. A. Pick your equation! B. E=hν or E=hc/λ Umm, I like things easy so I’ll use this one: E=hν

Planck’s Practice Problems 3. A photon of light produced by a surgical laser has an energy of 3. 027 x 10 -19 J. Calculate the frequency and the wavelength of the photon. A. Pick your equation! B. E=hν or E=hc/λ Umm, I like things easy so I’ll use this one: E=hν C. 3. 027 x 10 -19 J =(6. 626× 10 -34 Js)v

Planck’s Practice Problems 3. A photon of light produced by a surgical laser has an energy of 3. 027 x 10 -19 J. Calculate the frequency and the wavelength of the photon. A. Pick your equation! B. E=hν or E=hc/λ Umm, I like things easy so I’ll use this one: E=hν C. 3. 027 x 10 -19 J =(6. 626× 10 -34 Js)v D. v=(3. 027 x 10 -19 J)/(6. 626× 10 -34 Js)

Planck’s Practice Problems 3. A photon of light produced by a surgical laser has an energy of 3. 027 x 10 -19 J. Calculate the frequency and the wavelength of the photon. A. Pick your equation! B. E=hν or E=hc/λ Umm, I like things easy so I’ll use this one: E=hν C. 3. 027 x 10 -19 J =(6. 626× 10 -34 Js)v D. v=(3. 027 x 10 -19 J)/(6. 626× 10 -34 Js) v= 4. 568 x 1014 Hz (or 4. 568 x 1014/s or 4. 568 x 1014 s -1)

Planck’s Practice Problems 3. A photon of light produced by a surgical laser has an energy of 3. 027 x 10 -19 J. Calculate the frequency and the wavelength of the photon. A. Pick your equation! B. E=hν or E=hc/λ Umm, I like things easy so I’ll use this one: E=hν C. 3. 027 x 10 -19 J =(6. 626× 10 -34 Js)v D. v=(3. 027 x 10 -19 J)/(6. 626× 10 -34 Js) E. v=4. 568 x 10 -19 s -1 Now what? Solve for wavelength!

Planck’s Practice Problems 3. A photon of light produced by a surgical laser has an energy of 3. 027 x 10 -19 J. Calculate the frequency and the wavelength of the photon. A. Pick your equation! B. E=hν or E=hc/λ Umm, I like things easy so I’ll use this one: E=hν C. 3. 027 x 10 -19 J =(6. 626× 10 -34 Js)v D. v=(3. 027 x 10 -19 J)/(6. 626× 10 -34 Js) E. v=4. 568 x 10 -19 s -1 Now what? Solve for wavelength! F. c = λν or λ = c/ν

Planck’s Practice Problems 3. A photon of light produced by a surgical laser has an energy of 3. 027 x 10 -19 J. Calculate the frequency and the wavelength of the photon. A. Pick your equation! B. E=hν or E=hc/λ Umm, I like things easy so I’ll use this one: E=hν C. 3. 027 x 10 -19 J =(6. 626× 10 -34 Js)v D. v=(3. 027 x 10 -19 J)/(6. 626× 10 -34 Js) E. v=4. 568 x 10 -19 s -1 Now what? Solve for wavelength! F. c = λν or λ = c/ν G. λ=(3. 00× 108 m/s) / 4. 568 x 10 -19 s -1

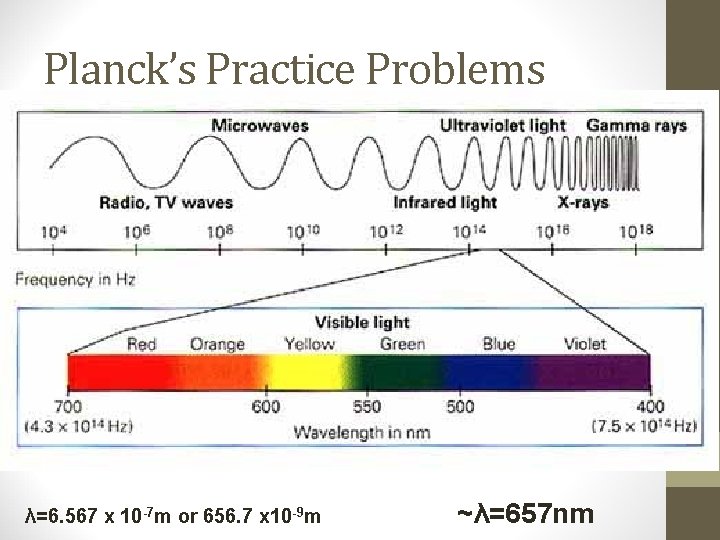
Planck’s Practice Problems 3. A photon of light produced by a surgical laser has an energy of 3. 027 x 10 -19 J. Calculate the frequency and the wavelength of the photon. A. Pick your equation! B. E=hν or E=hc/λ Umm, I like things easy so I’ll use this one: E=hν C. 3. 027 x 10 -19 J =(6. 626× 10 -34 Js)v D. v=(3. 027 x 10 -19 J)/(6. 626× 10 -34 Js) E. v=4. 568 x 10 -19 s -1 Now what? Solve for wavelength! F. c = λν or λ = c/ν G. λ=(3. 00× 108 m/s) / 4. 568 x 10 -19 s -1 λ=6. 567 x 10 -7 m or 656. 7 x 10 -9 m ~λ=657 nm

Planck’s Practice Problems 3. A photon of light produced by a surgical laser has an energy of 3. 027 x 10 -19 J. Calculate the frequency and the wavelength of the photon. A. Pick your equation! B. E=hν or E=hc/λ Umm, I like things easy so I’ll use this one: E=hν C. 3. 027 x 10 -19 J =(6. 626× 10 -34 Js)v D. v=(3. 027 x 10 -19 J)/(6. 626× 10 -34 Js) E. v=4. 568 x 10 -19 s -1 Now what? Solve for wavelength! F. c = λν or λ = c/ν G. λ=(3. 00× 108 m/s) / 4. 568 x 10 -19 s -1 λ=6. 567 x 10 -7 m or 656. 7 x 10 -9 m ~λ=657 nm

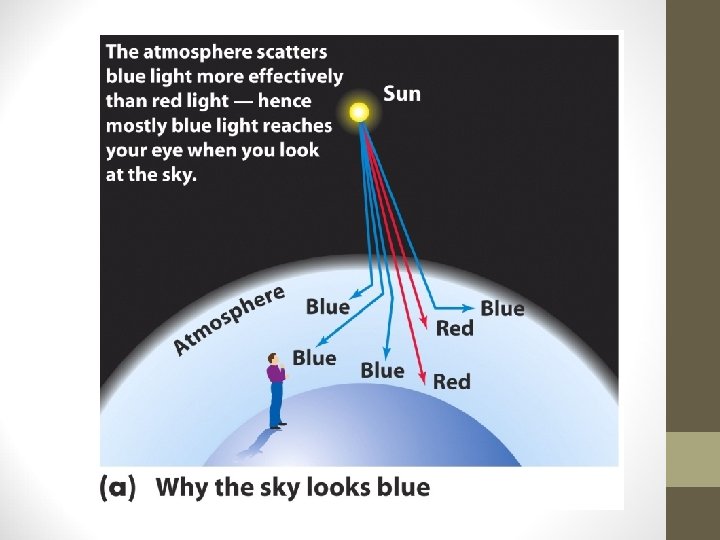
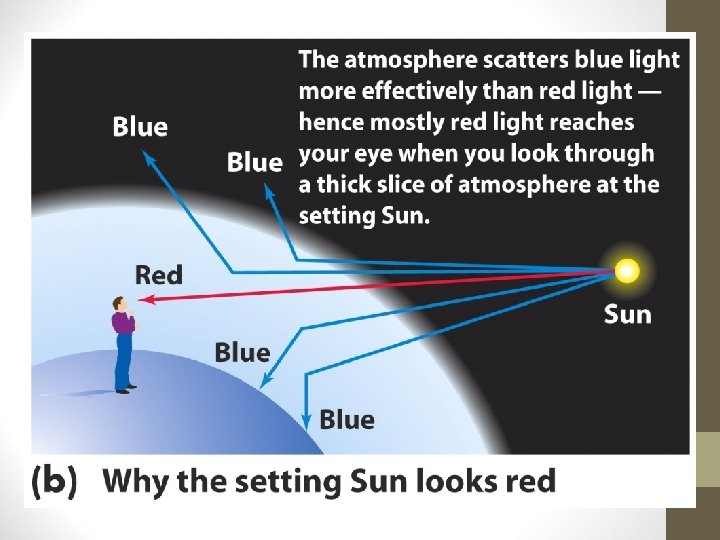
For funsies • Why is the sky blue? • Why are sunsets red?

For funsies • Why is the sky blue? • Why are sunsets red? • Blue is scattered more than other colors because it travels as shorter, smaller waves. • As the sunlight has passed through all this air, the air molecules have scattered and rescattered the blue light many times in many directions. • BUT… • The surface of Earth has reflected and scattered the light. All this scattering mixes the colors back together again so we see more white and less blue.


For funsies • Why is the sky blue? • Why are sunsets red? • At sunset, even more of the blue light is scattered, allowing the reds and yellows to pass straight through to your eyes.
