Electromagnetic Radiation Electromagnetic Spectrum Light 1600s sunlight considered


















- Slides: 32

Electromagnetic Radiation

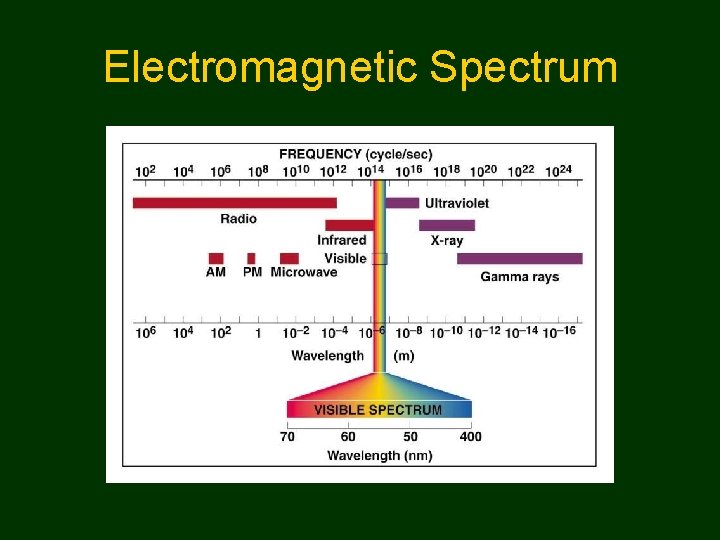
Electromagnetic Spectrum

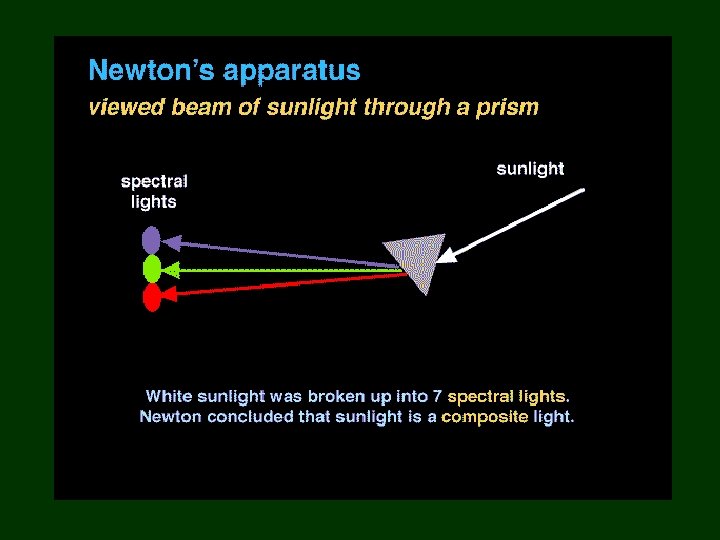
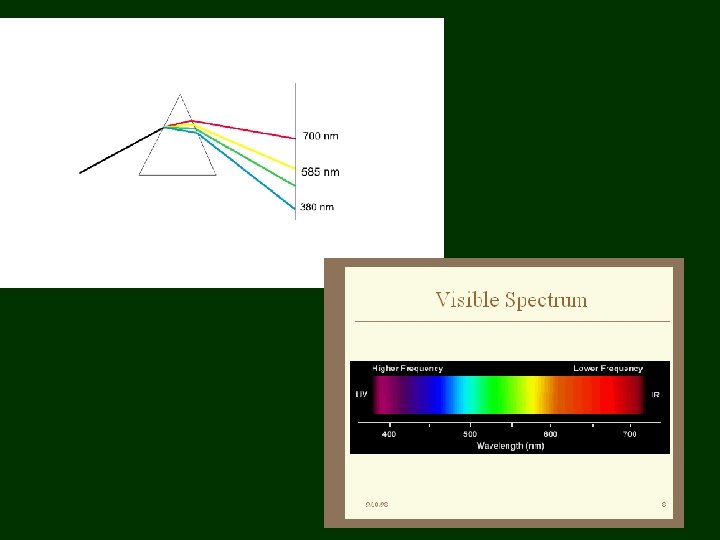
Light 1600’s – sunlight considered purest form of light • 1665 – Isaac Newton – passed a beam of sunlight through a prism • beam spread out • band of colors (Roy G. Biv) – rejoined the colors with 2 nd prism & got white light again

Notice: red light bent the least, violet the most


Newton Thought light made up of tiny particles with no mass – explains why shadows have sharp edges • Couldn’t explain how particles of different colors were different or why were refracted differently by prism • Couldn’t explain why 2 beams of light didn’t affect each other when crossed – particles of light should collide off each other

Christian Huygens 1678: suggested light composed of waves • explained why 2 beams of light could cross each other without being disturbed • explained refraction in prism: - different colors have different wavelengths • people were used to waves in water – water waves move around an obstruction – waves couldn’t explain shadows with sharp edges

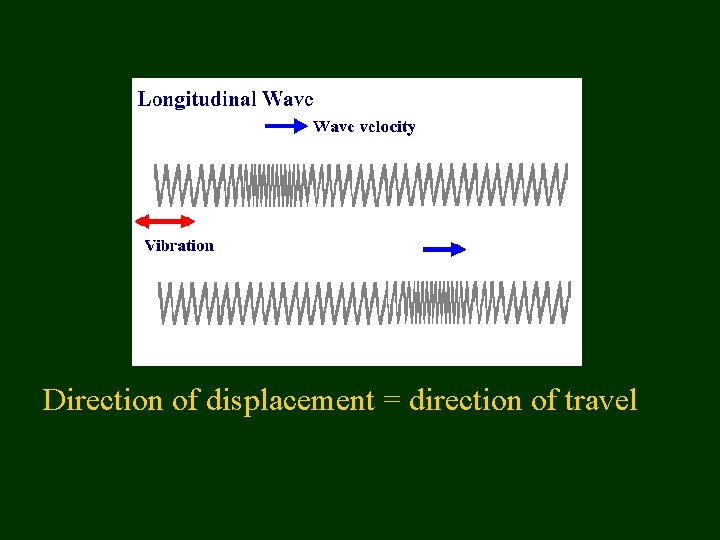
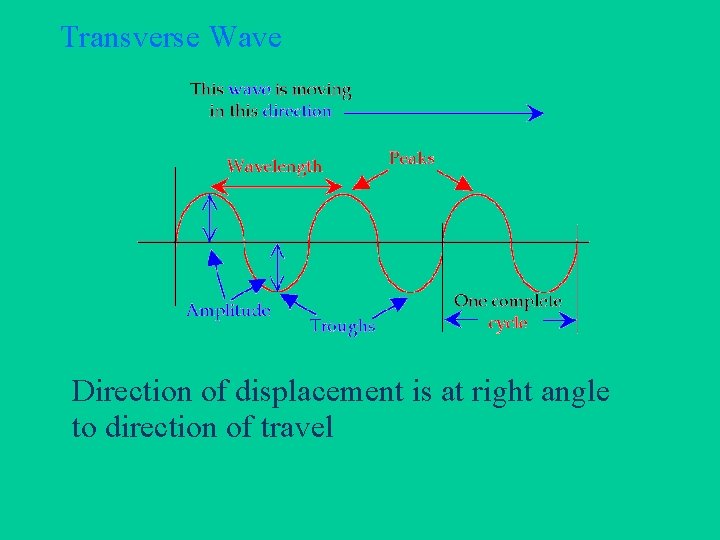
Wave Vocabulary • • • Transverse and Longitudinal Wavelength Frequency Amplitude Velocity

Direction of displacement = direction of travel

Transverse Wave Direction of displacement is at right angle to direction of travel

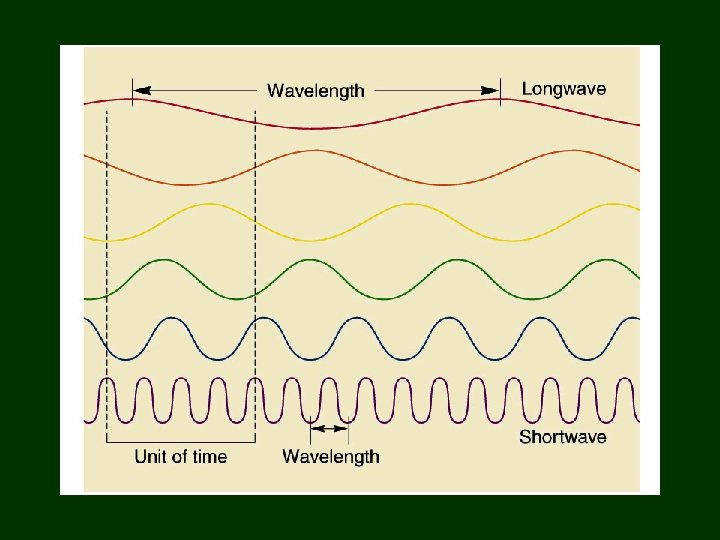
Wavelength vs. Frequency • WAVELENGTH = distance light travels to complete 1 cycle • FREQUENCY = number of cycles completed in 1 second


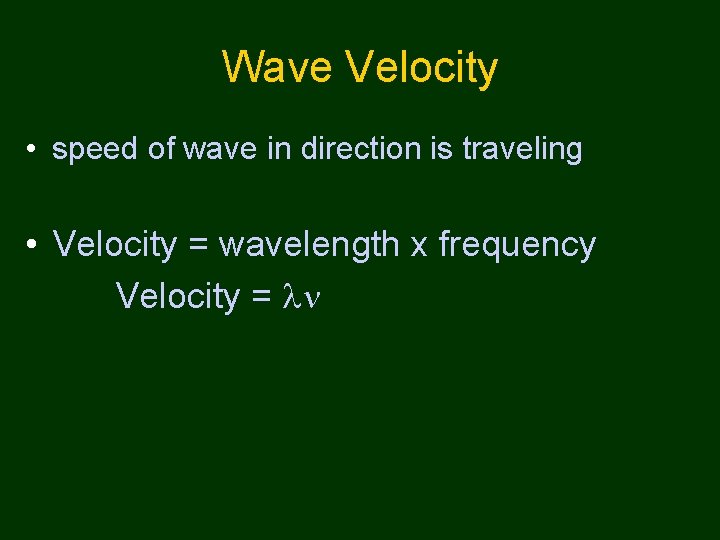
Wave Velocity • speed of wave in direction is traveling • Velocity = wavelength x frequency Velocity =

Light: Particles vs. Waves Huygens had better argument, but Newton was more famous - people went with Newton's theory: Light was particles! • Speed of light 1 st determined about 1676 by Danish astronomer – speed of light = 3. 0 X 108 m/sec

Calculating Wavelengths of Light • visible light waves have lengths ~ 1/20, 000 cm – Red a little longer, violet a little shorter • short wavelength explains why light cast sharp shadows despite being waves – waves can only bend around obstacles that are about same length – cannot bend around anything substantially longer than itself

The Ether • Water waves move in water • Sound waves move in air • Light waves move through vacuum – Gravity, Electricity, & Magnetism also felt across vacuum • people couldn't accept this: – postulated subtle form of matter called ether – not easily detectable • “Ether” idea held until 1900

Maxwell • 1864 -1873 worked out equations describing electricity & magnetism • electric & magnetic fields cannot exist independently • electromagnetic radiation in Maxwell’s equation moved at same speed as light! – could not be coincidence!

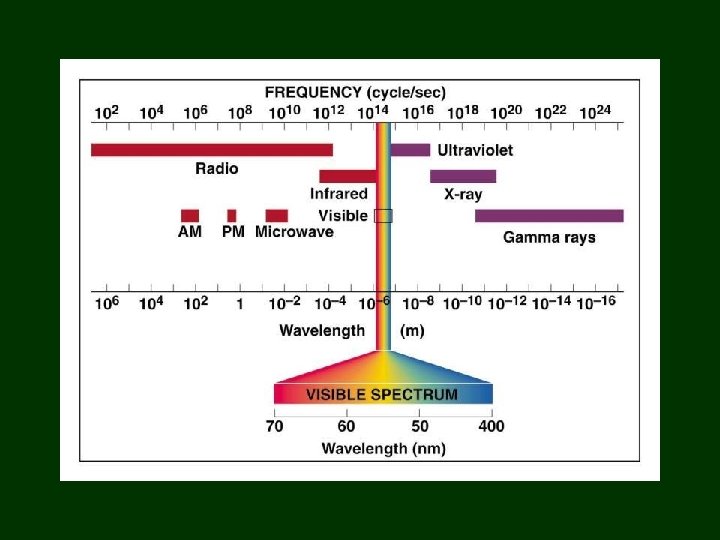
Light that can’t be seen? • Light was electromagnetic radiation! • Maxwell’s equations unified electricity, magnetism, & light • But visible light only accounts for a fraction of Maxwell’s equations – ? other frequencies and wavelengths

Heat & Light: Separate or same? Herschel – 1800 studied spectrum with thermometer - found highest temperature at red end & coolest at violet end – placed thermometer beyond red & temp was higher there than anywhere in visible spectrum – discovered Infared rays, which we cannot see


Infrared Radiation • By 1850: infrared rays were demonstrated to have all the properties of light – except they could not be seen by eye

Silver Nitrate as detector • 1614: knew that silver nitrate (white cmpd: Ag. NO 3) darkens on exposure to sunlight • 1770: Scheele soaked strips of white paper in Ag. NO 3 solutions and placed them in different parts of spectrum – darkened least quickly in red and fastest in violet • ? the first photographs

Discovery of ultraviolet! • After Herschel discovered infrared using thermometers: – 1801: Ritter repeated Scheele’s experiment with paper soaked in Ag. NO 3 & put strips beyond violet – strips darkened even quicker than in violet light – discovered ultraviolet light!

Radio Waves • 1888: Hertz used oscillating electric current to emit electromagnetic radiation – had detector that could move around to map the electromagnetic wave & determine its length • found radio waves far beyond infrared radiation – have wavelengths from cm to km

X-Rays • 1895: Roentgen discovered that his cathode ray tube was emitting radiation = X-rays


Electromagnetic Spectrum

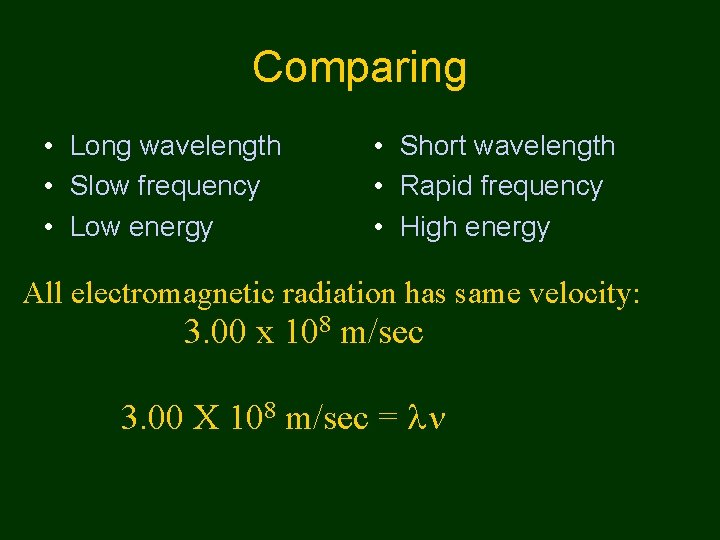
Comparing • Long wavelength • Slow frequency • Low energy • Short wavelength • Rapid frequency • High energy All electromagnetic radiation has same velocity: 3. 00 x 108 m/sec 3. 00 X 108 m/sec =

Heat Flow • Objects hotter than surroundings lose heat as electromagnetic radiation – higher the temp, the more intense the radiation • Hot objects glow! – glow different colors at different temperatures!

Ultraviolet Catastrophe • Classical physics - Assume that every wavelength has equal chance of being radiated • Classical wave model could not explain why different colors were emitted at different temperatures

Planck • shorter the wavelength, the less chance it has to be emitted! • Matter can gain or release energy only in very small increments = quanta

What physical explanation goes with Planck’s assumption? • Pre-Planck: – Energy was considered to be continuous – could be broken into smaller & smaller bits indefinitely • Planck: – Energy consists of tiny particles that can’t be divided into anything smaller