Electrolytic Cells Lab Lesson 10 Electrolytic cell Lab

Electrolytic Cells Lab Lesson 10
Electrolytic cell Lab Dec 17, 2008 Purpose: Full Name To draw and completely analyze five electrolytic cells. Procedure: 1. Fold a filter paper and place in a watch glass 2. Add 20 m. L of electrolyte 3. Immerse the carbon electrodes in the electrolyte to complete the electrolytic cell. Data:
1. KBr(aq) electrolytic cell with phenolphthalein. Cathode: Anode: Observations: Significance: e- - C Power Source + e- + C
1. KBr(aq) electrolytic cell with phenolphthalein. . Cathode: Observations: Significance: e- - C Anode: Power Source + K+ Br. H 2 O e- + C
1. KBr(aq) electrolytic cell with phenolphthalein. Cathode: Anode: Observations: Significance: e- - C Power Source + K+ Br. H 2 O e- + C
1. KBr(aq) electrolytic cell with phenolphthalein. Cathode: Observations: bubbles Significance: e- - C Anode: Power Source + K+ Br. H 2 O e- + C
1. KBr(aq) electrolytic cell with phenolphthalein. Cathode: Observations: bubbles Significance: e- - C Anode: Power Source + K+ Br. H 2 O e- + C
1. KBr(aq) electrolytic cell with phenolphthalein. Cathode: Observations: bubbles Significance: e- - C Anode: pink Power Source + K+ Br. H 2 O e- + C
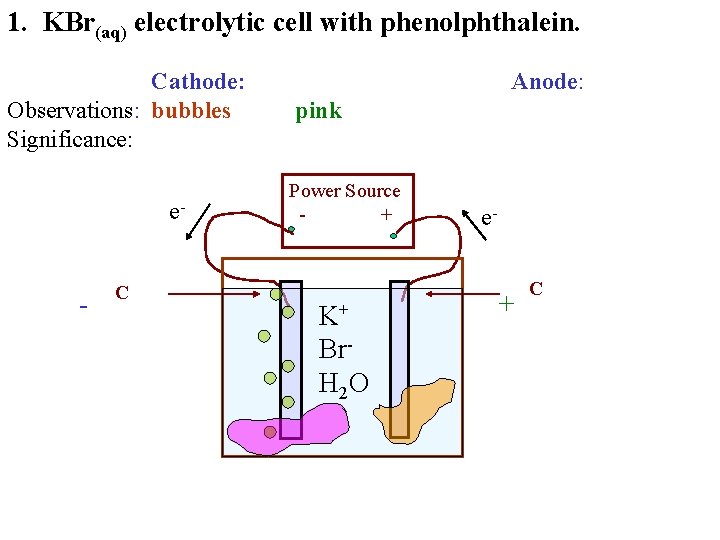
1. KBr(aq) electrolytic cell with phenolphthalein. Cathode: Observations: bubbles Significance: e- - C Anode: pink Power Source + K+ Br. H 2 O e- + C
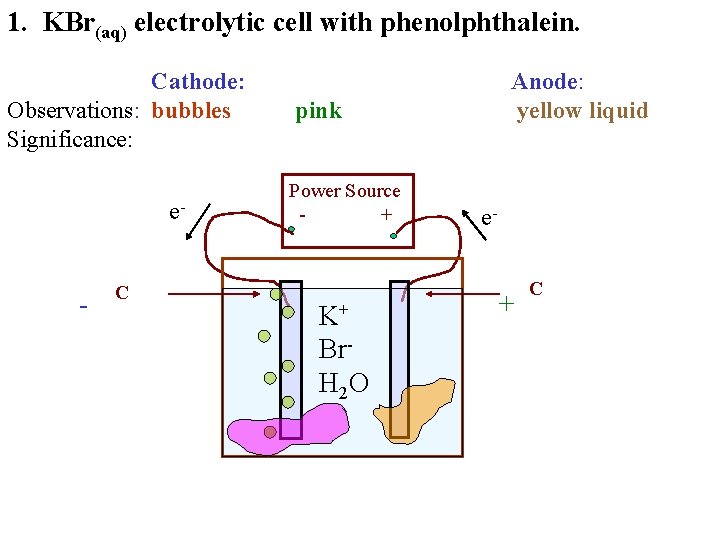
1. KBr(aq) electrolytic cell with phenolphthalein. Cathode: Observations: bubbles Significance: e- - C Anode: yellow liquid pink Power Source + K+ Br. H 2 O e- + C
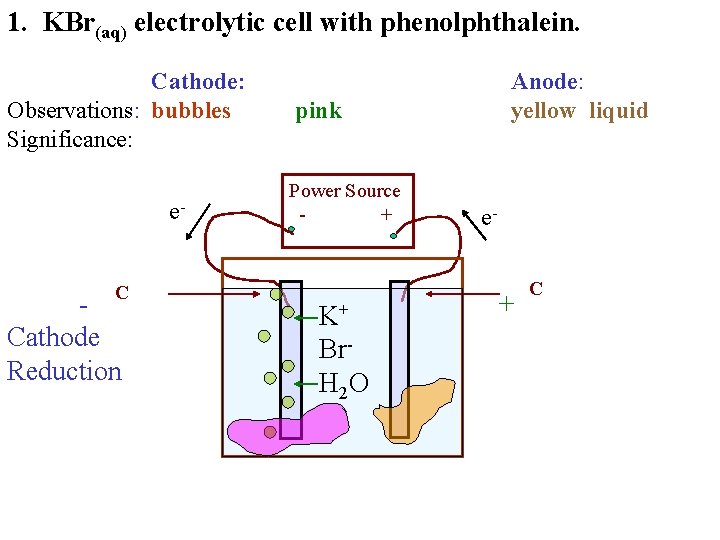
1. KBr(aq) electrolytic cell with phenolphthalein. Cathode: Observations: bubbles Significance: e. C Cathode Reduction Anode: yellow liquid pink Power Source + K+ Br. H 2 O e- + C
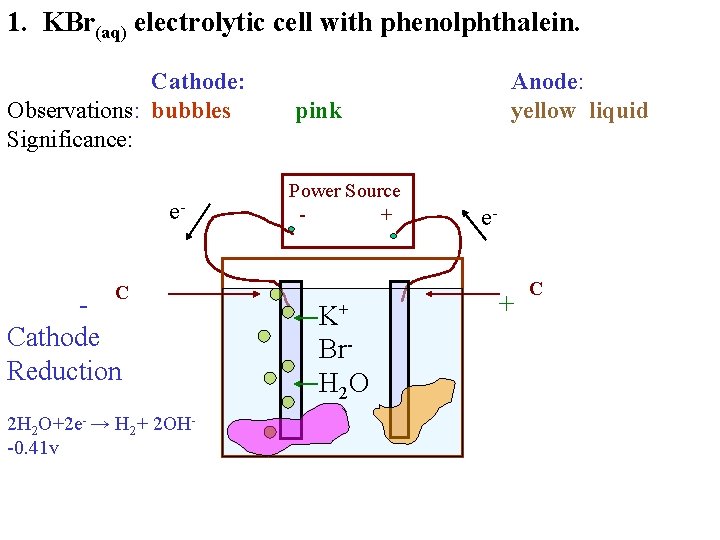
1. KBr(aq) electrolytic cell with phenolphthalein. Cathode: Observations: bubbles Significance: e. C Cathode Reduction 2 H 2 O+2 e- → H 2+ 2 OH-0. 41 v Anode: yellow liquid pink Power Source + K+ Br. H 2 O e- + C
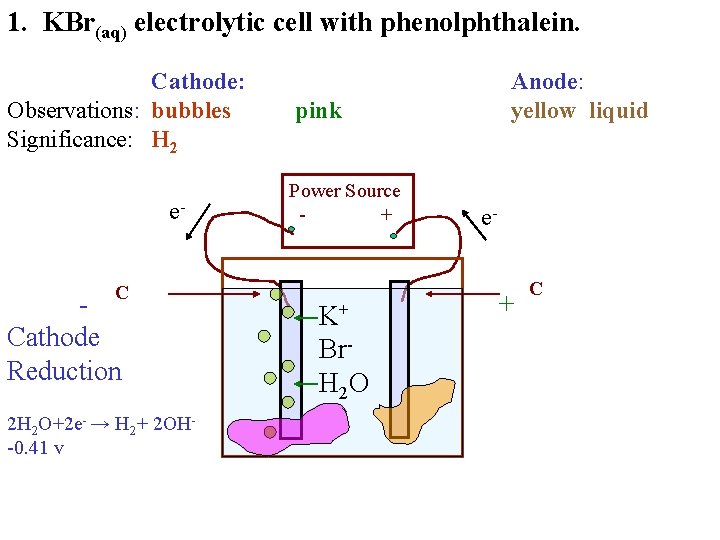
1. KBr(aq) electrolytic cell with phenolphthalein. Cathode: Observations: bubbles Significance: H 2 e. C Cathode Reduction 2 H 2 O+2 e- → H 2+ 2 OH-0. 41 v Anode: yellow liquid pink Power Source + K+ Br. H 2 O e- + C
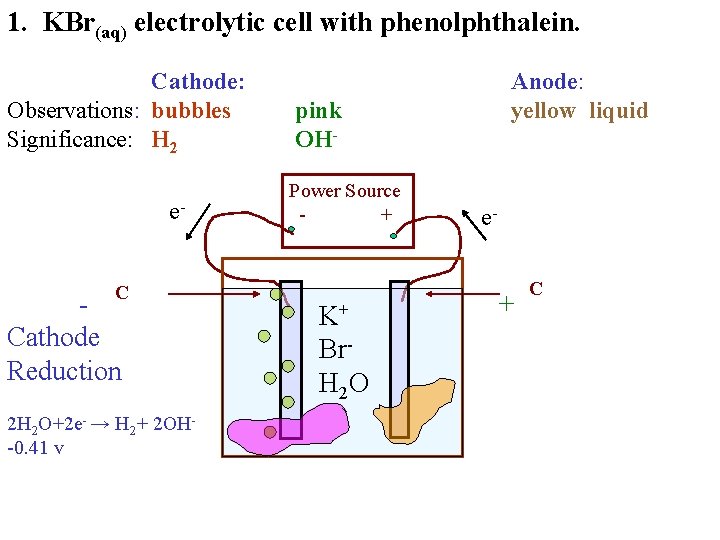
1. KBr(aq) electrolytic cell with phenolphthalein. Cathode: Observations: bubbles Significance: H 2 e. C Cathode Reduction 2 H 2 O+2 e- → H 2+ 2 OH-0. 41 v Anode: yellow liquid pink OHPower Source + K+ Br. H 2 O e- + C
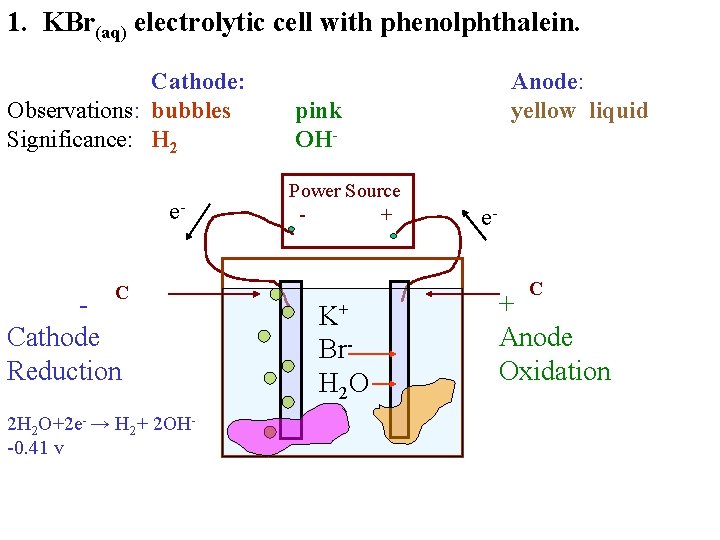
1. KBr(aq) electrolytic cell with phenolphthalein. Cathode: Observations: bubbles Significance: H 2 e. C Cathode Reduction 2 H 2 O+2 e- → H 2+ 2 OH-0. 41 v Anode: yellow liquid pink OHPower Source + e. C K+ Br. H 2 O + Anode Oxidation
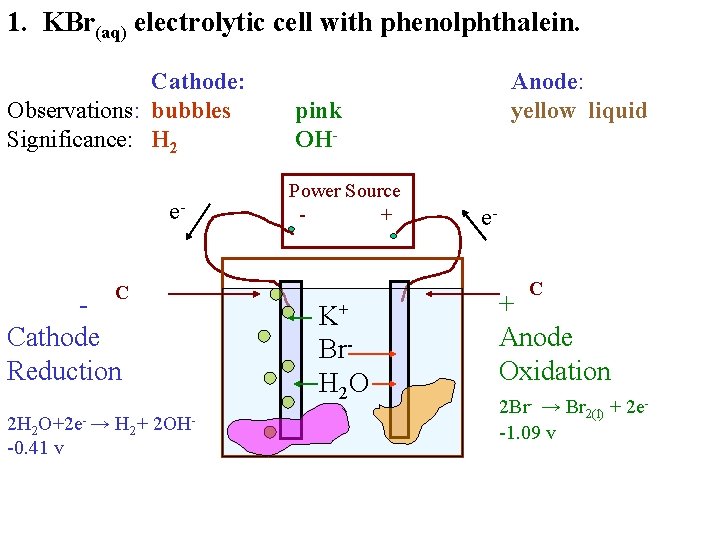
1. KBr(aq) electrolytic cell with phenolphthalein. Cathode: Observations: bubbles Significance: H 2 e. C Cathode Reduction 2 H 2 O+2 e- → H 2+ 2 OH-0. 41 v Anode: yellow liquid pink OHPower Source + e. C K+ Br. H 2 O + Anode Oxidation 2 Br- → Br 2(l) + 2 e-1. 09 v
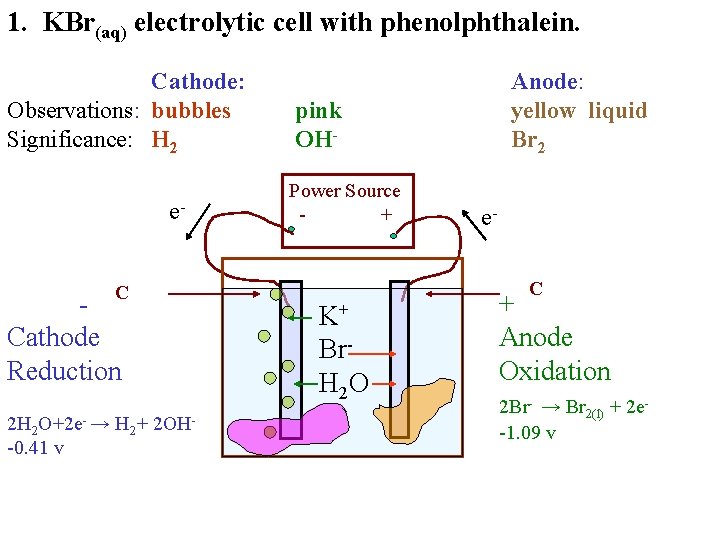
1. KBr(aq) electrolytic cell with phenolphthalein. Cathode: Observations: bubbles Significance: H 2 e. C Cathode Reduction 2 H 2 O+2 e- → H 2+ 2 OH-0. 41 v Anode: yellow liquid Br 2 pink OHPower Source + e. C K+ Br. H 2 O + Anode Oxidation 2 Br- → Br 2(l) + 2 e-1. 09 v
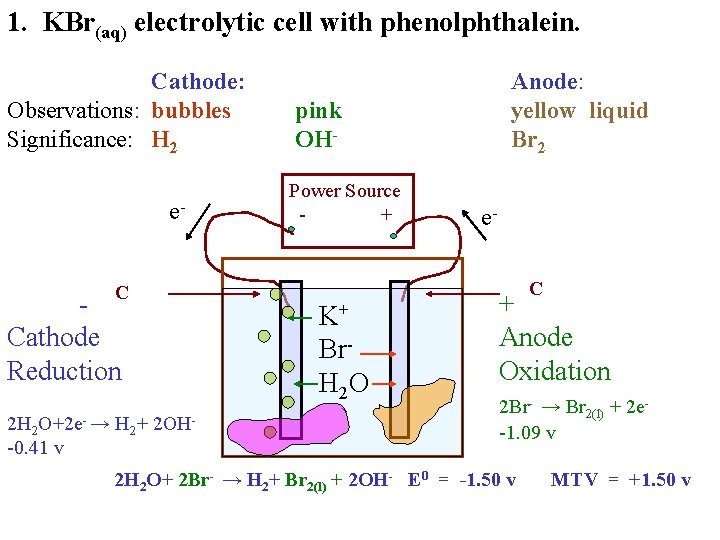
1. KBr(aq) electrolytic cell with phenolphthalein. Cathode: Observations: bubbles Significance: H 2 e. C Cathode Reduction 2 H 2 O+2 e- → H 2+ 2 OH-0. 41 v Anode: yellow liquid Br 2 pink OHPower Source + e. C K+ Br. H 2 O + Anode Oxidation 2 Br- → Br 2(l) + 2 e-1. 09 v 2 H 2 O+ 2 Br- → H 2+ Br 2(l) + 2 OH- E 0 = -1. 50 v MTV = +1. 50 v
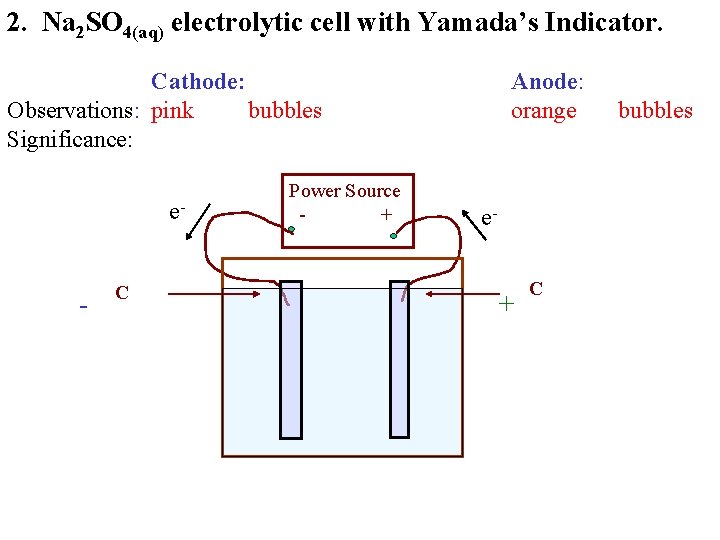
2. Na 2 SO 4(aq) electrolytic cell with Yamada’s Indicator. Cathode: Observations: pink bubbles Significance: e- - C Power Source + Anode: orange e- + C bubbles
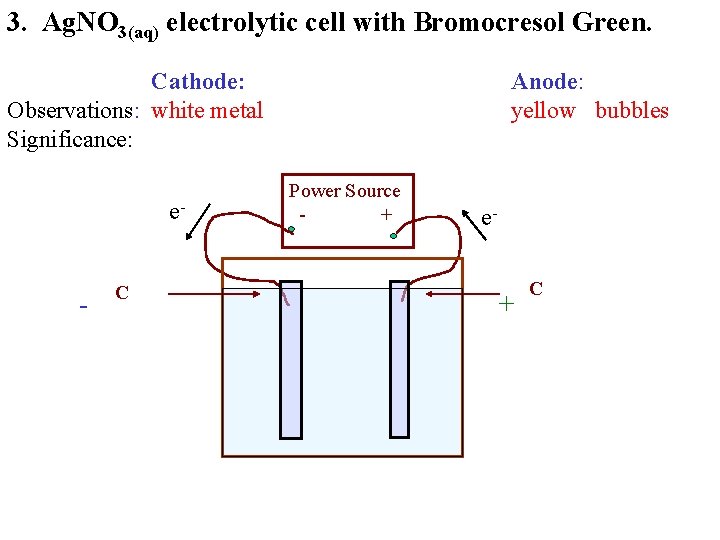
3. Ag. NO 3(aq) electrolytic cell with Bromocresol Green. Cathode: Observations: white metal Significance: e- - C Anode: yellow bubbles Power Source + e- + C
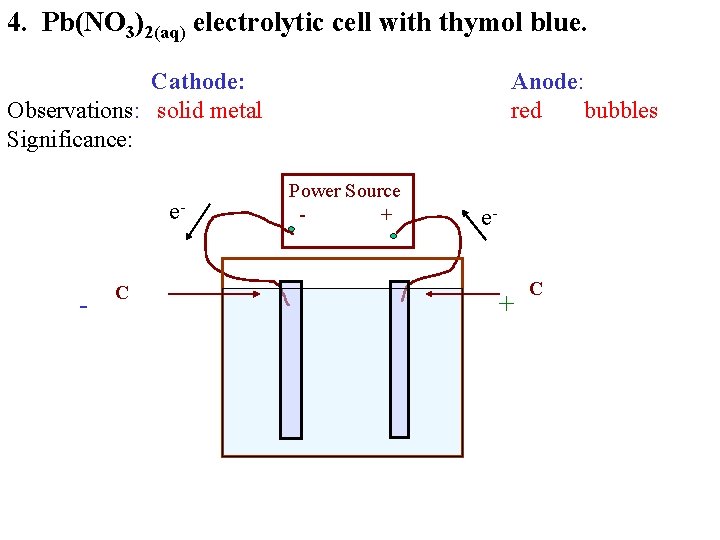
4. Pb(NO 3)2(aq) electrolytic cell with thymol blue. Cathode: Observations: solid metal Significance: e- - C Anode: red bubbles Power Source + e- + C
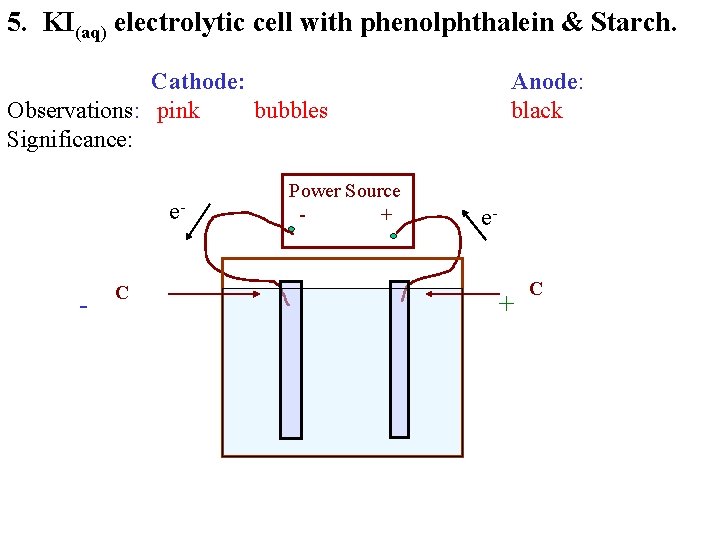
5. KI(aq) electrolytic cell with phenolphthalein & Starch. Cathode: Observations: pink bubbles Significance: e- - C Power Source + Anode: black e- + C

Conclusion State that you did what you set out to do in the purpose. Define an electrolytic cell. Do I need to say, do not use pronouns, Navreen? Start with the main rule and describe how you would completely analyze an electrolytic cell- use your notes, don't be a hero! Discuss how you determined the anode, cathode, site of oxidation, and site of reduction. Discuss electron flow and ion migration. Describe how you determined the anode and cathode reaction and include a discussion of the overpotential effect for water. Describe what electrolytic cells are used for and list all of the chemicals produced in this lab.
- Slides: 23