Electrolytic Cells Electrolytic Cells Use electricity to force








- Slides: 17

Electrolytic Cells

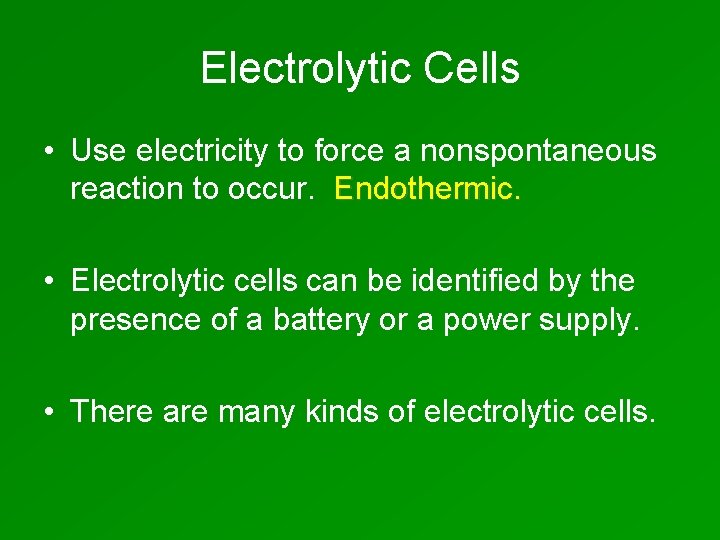
Electrolytic Cells • Use electricity to force a nonspontaneous reaction to occur. Endothermic. • Electrolytic cells can be identified by the presence of a battery or a power supply. • There are many kinds of electrolytic cells.

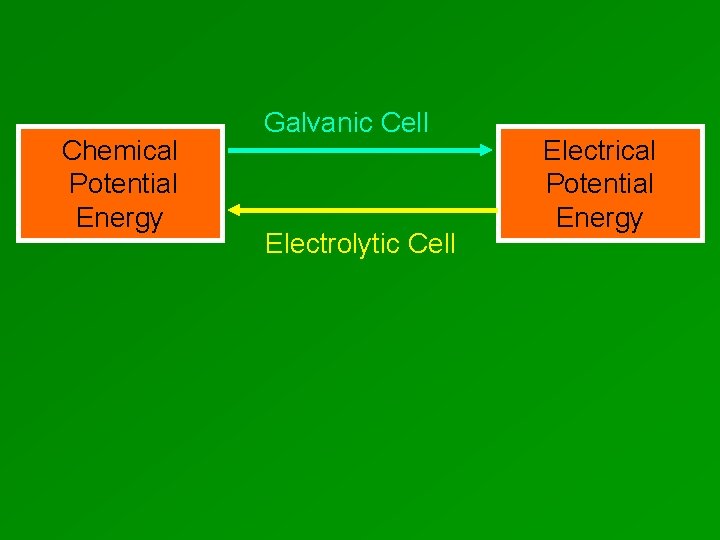
Chemical Potential Energy Galvanic Cell Electrolytic Cell Electrical Potential Energy

Electrolytic Decomposition of H 2 O H 2 is produced at one electrode, O 2 at the other. 2 H 2 O + energy 2 H 2 + O 2 Can you tell from the picture which electrode is producing H 2?

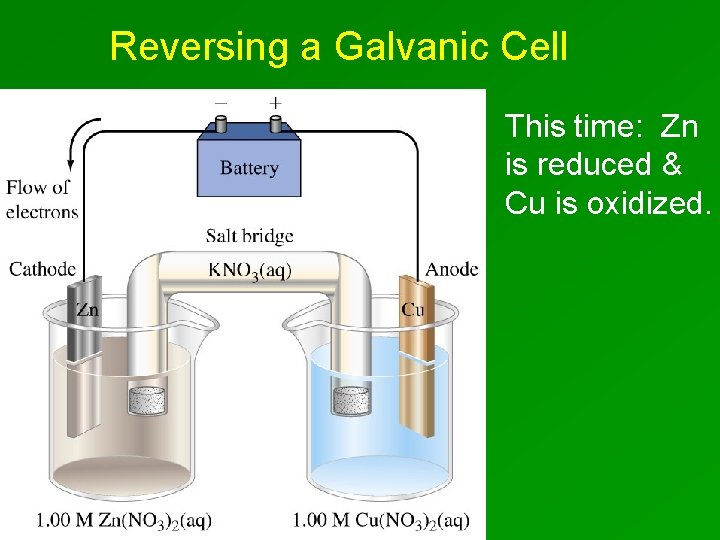
Reversing a Galvanic Cell This time: Zn is reduced & Cu is oxidized.

Two kinds of electrolytic cells • Fused Salt Cells – Preparation of pure metals (Fused means melted!) • Plating Cells – Designing surface to have specific properties

Fused Salt Cells • Used to purify metals from their ores.

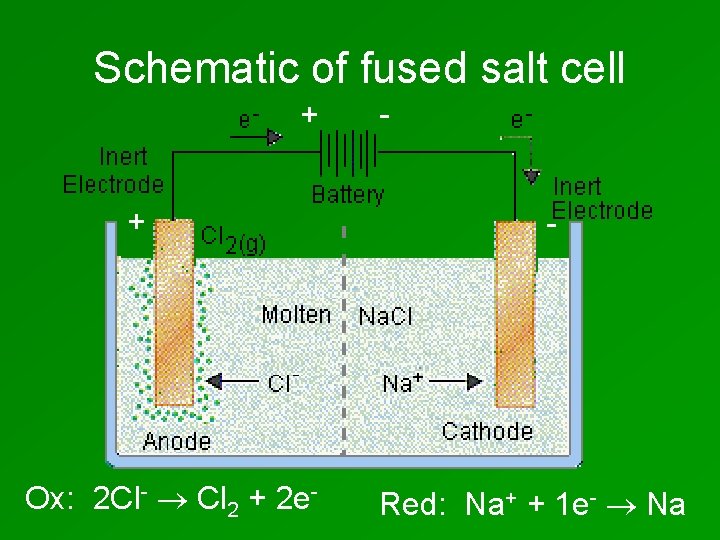
Schematic of fused salt cell + + Ox: 2 Cl- Cl 2 + 2 e- - Red: Na+ + 1 e- Na

• Why does the Na. Cl have to be molten? • Which electrode will the Na+ ions be attracted to? • Which electrode will the Cl- ions be attracted to?

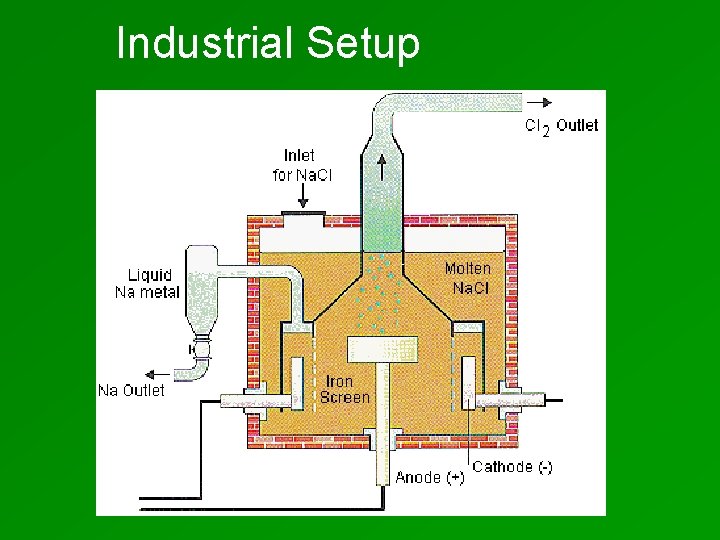
Industrial Setup


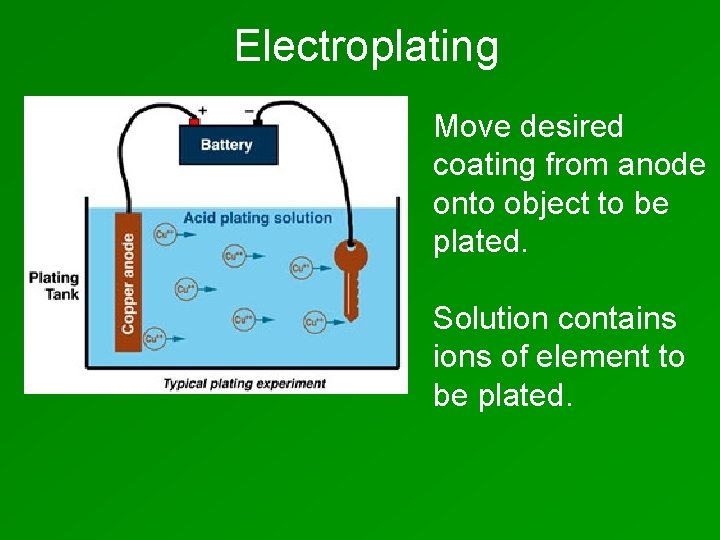
Electroplating Move desired coating from anode onto object to be plated. Solution contains ions of element to be plated.

Electroplating: Cu onto Al • Al is above Cu in Table J. • This reaction will not happen spontaneously. • Use an external energy source – a power supply or a battery – to force reaction to occur.

An Ox ate a Red Cat • Anode is still electrode at which oxidation occurs. • Cathode is still electrode at which reduction occurs. • Polarity of electrodes is different! – Anode is positive. – Cathode is negative.

A POX on Electrolytic Cells. • Anode – Positive – Oxidation • In an electrolytic cell, the polarity is determined by the outside power supply. • The anode is hooked up to the positive terminal and the cathode is hooked up to the negative terminal. • Look at drawings in Regents questions.

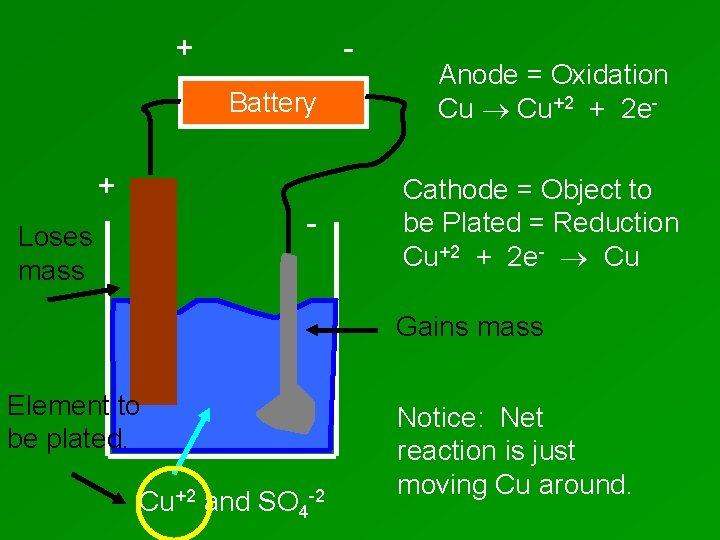
+ Battery + - Loses mass Anode = Oxidation Cu Cu+2 + 2 e. Cathode = Object to be Plated = Reduction Cu+2 + 2 e- Cu Gains mass Element to be plated. Cu+2 and SO 4 -2 Notice: Net reaction is just moving Cu around.

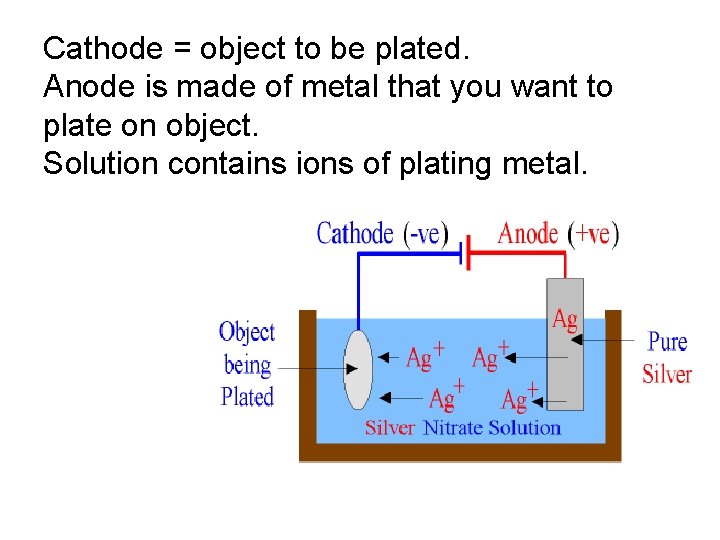
Cathode = object to be plated. Plating Anode is made of metal that you want to plate on object. Solution contains ions of plating metal.