Electrolysis What is electrolysis Electrolysis keywords means Anode



- Slides: 14

Electrolysis

What is electrolysis? Electrolysis • keywords means Anode – positive • • “breaking up a electrode compound with electricity” • Cathode – negative electrode Ion -the charged • • From Greekparticle • Electrolyte – substance can be electrolysed • Electro –that electricity • Lysis – breaking up • Anion – ion that goes to the anode • Cation – ion that goes to the cathode

What is electrolysis? • Electrolysis only works on substances made of ions. • These are mostly the compounds of metals. • The products of electrolysis are vital for our industry.

What is electrolysis? • Electrolysis will only work if the ions in the substances are free to move. • In solids the ions are fixed in place, so electrolysis will not work on solids • But • Electrolysis works on liquids and molten compounds • Electrolysis works on solutions.

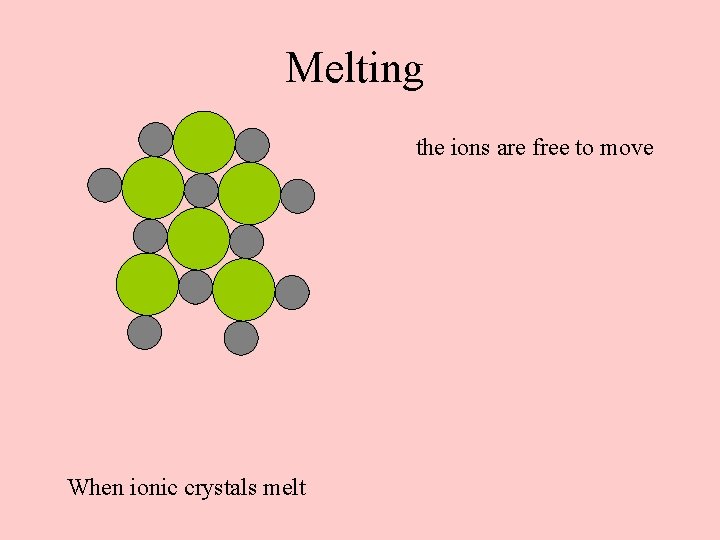
Melting the ions are free to move When ionic crystals melt

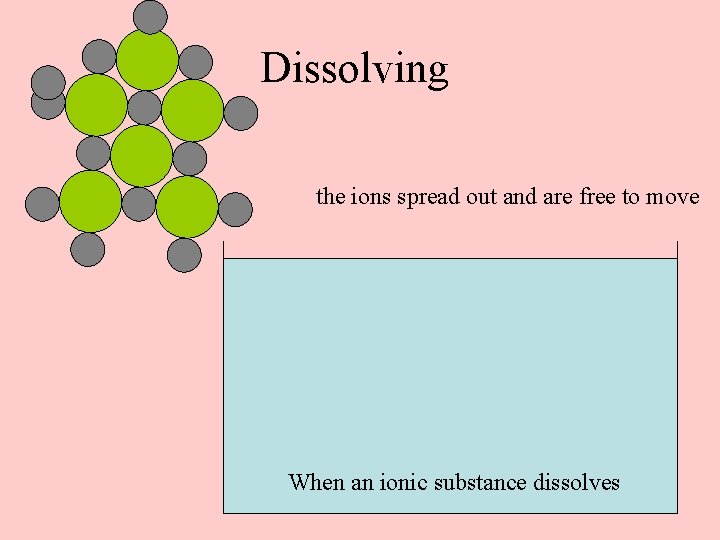
Dissolving the ions spread out and are free to move When an ionic substance dissolves

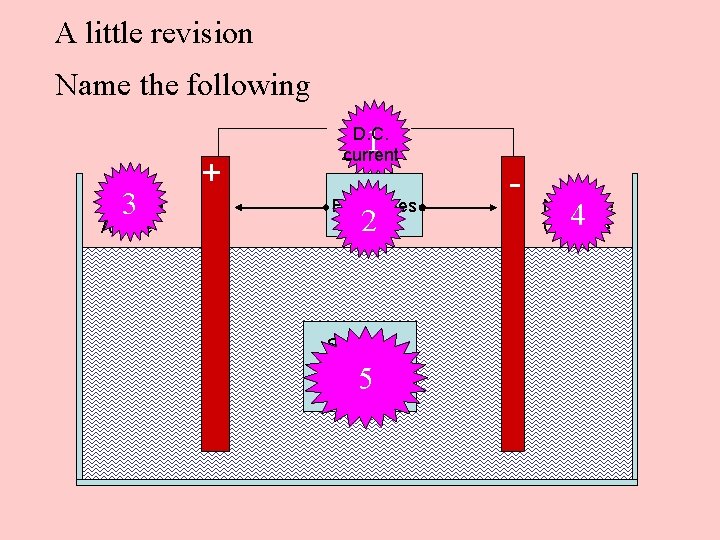
A little revision Name the following 3 Positive Anode + 1 D. C. current Electrodes 2 Sodium Chloride Solution 5 - Negative Cathode 4

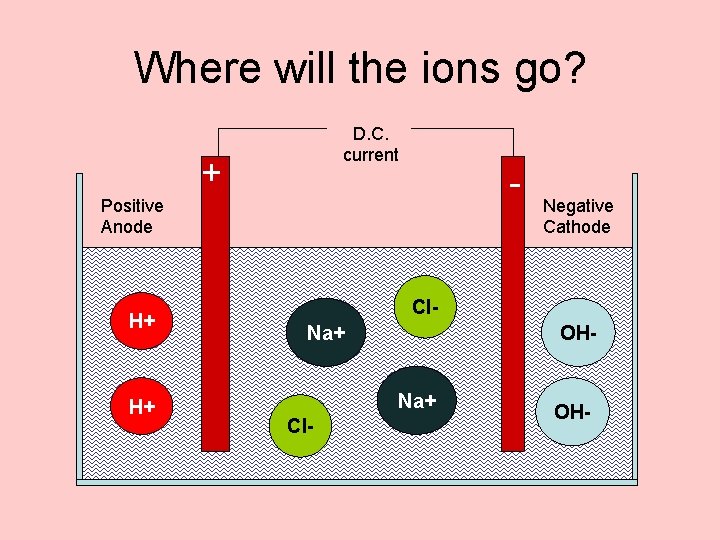
When Salt Dissolves • The sodium ions and • Some water the chloride ions split molecules also split up. up • This gives us • Na+ ions • H+ ions • and • Cl- ions • OH- ions

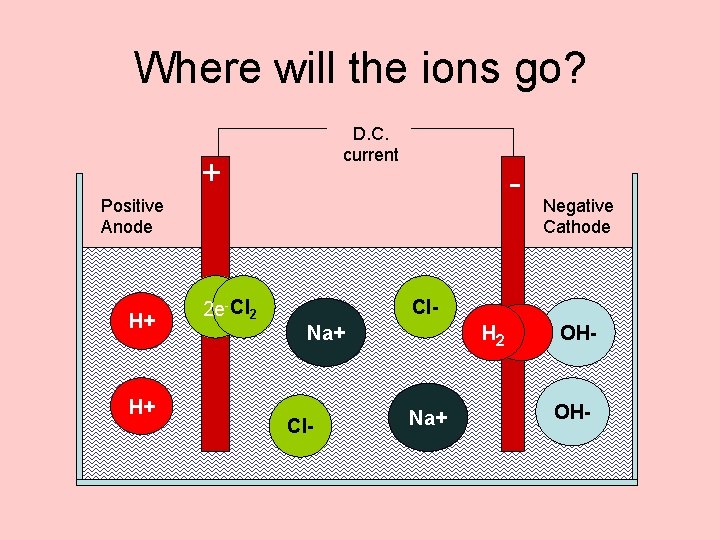
Where will the ions go? D. C. current + - Positive Anode H+ H+ Negative Cathode Cl. Na+ OHNa+ Cl- OH-

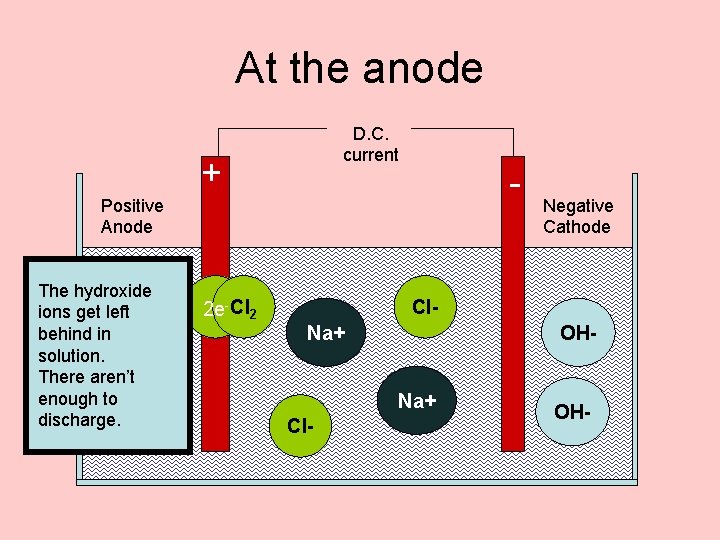
At the anode D. C. current + - Positive Anode chloride The hydroxide There are lots of loseleft chloride ions get ions in H+ electrons brine. in and behind become They arechlorine solution. gas attracted There aren’t to the anode enough first to 2 Cl- Cl 2 +H+ 2 edischarge. OXIDATION 2 e- Cl 2 Negative Cathode Cl. Na+ OHNa+ Cl- OH-

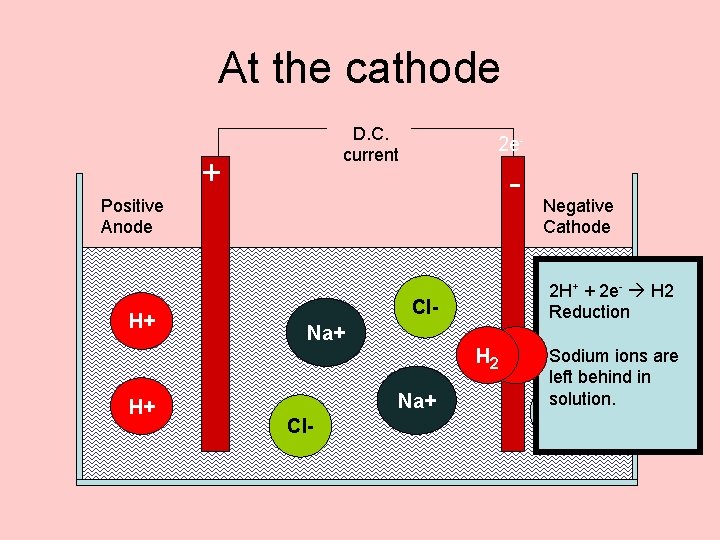
At the cathode D. C. current + 2 e- - Positive Anode H+ Cl. Na+ H 2 H+ Na+ Cl- Negative Cathode 2 H 2 e- H 2 The+ + Hydrogen electrons ions get to Reduction pumped the into OHthe cathode. before the sodium Sodium ions. are Positive left behind ions in are attracted togas Hydrogen solution. it. is OHdischarged.

Where will the ions go? D. C. current + - Positive Anode H+ H+ 2 e- Cl 2 Negative Cathode Cl. Na+ Cl- H 2 Na+ OH-

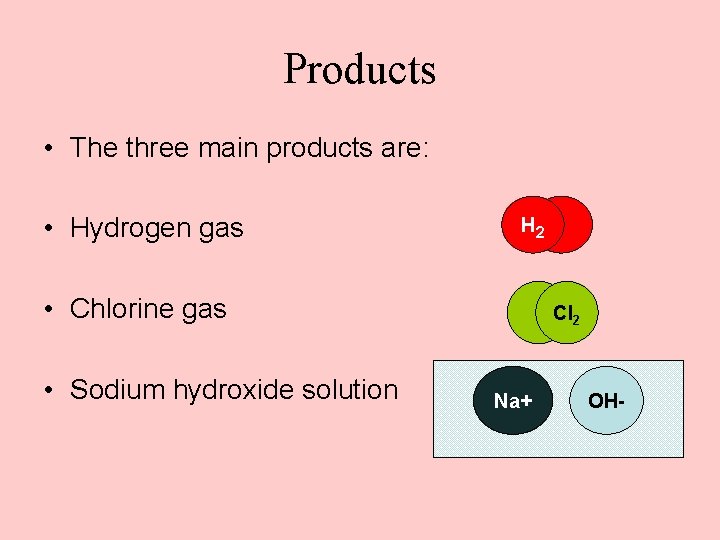
Products • The three main products are: • Hydrogen gas H 2 • Chlorine gas • Sodium hydroxide solution Cl 2 Na+ OH-

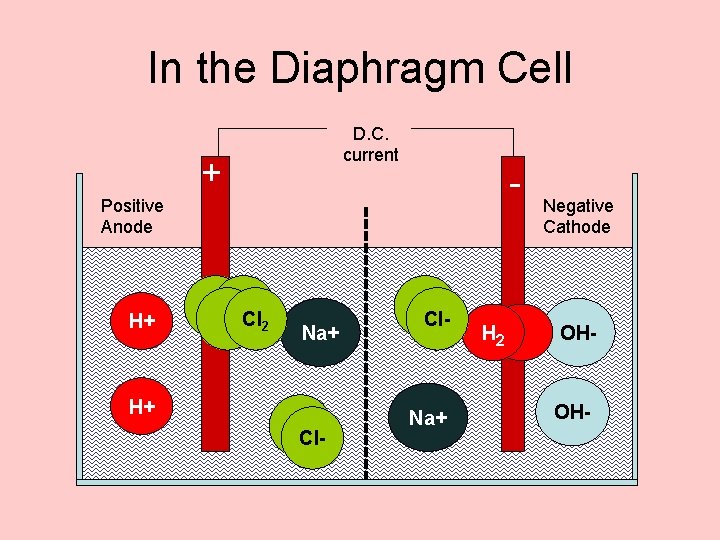
In the Diaphragm Cell D. C. current + - Positive Anode H+ H+ 2 e- Cl 2 Na+ Cl. Cl- Na+ H 2 Negative Cathode OH-