ELECTROLYSIS EXPLAIN THE PROCESS OF ELECTROLYSIS AND ITS





- Slides: 12

ELECTROLYSIS EXPLAIN THE PROCESS OF ELECTROLYSIS AND ITS USES

ELECTROLYSIS • A process where electrical energy is transformed into chemical energy • It is not spontaneous, electrical energy must be supplied for a reaction to occur It has important applications such as: • Electroplating • Extraction of reactive metals such as Na, Al from metal ore • Industrial production of Na. OH, Cl 2, H 2 • Recharging of car batteries & other rechargeable cells • Refining of copper metals

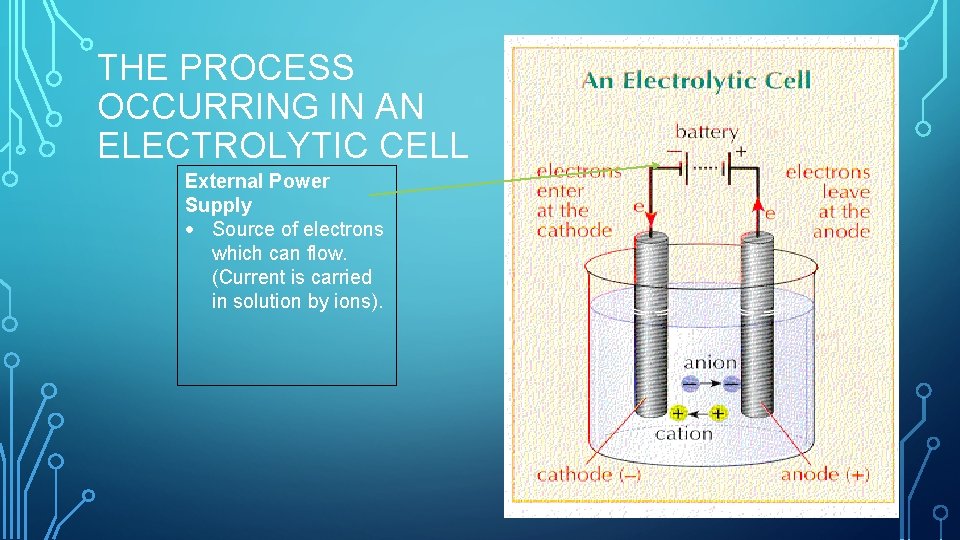
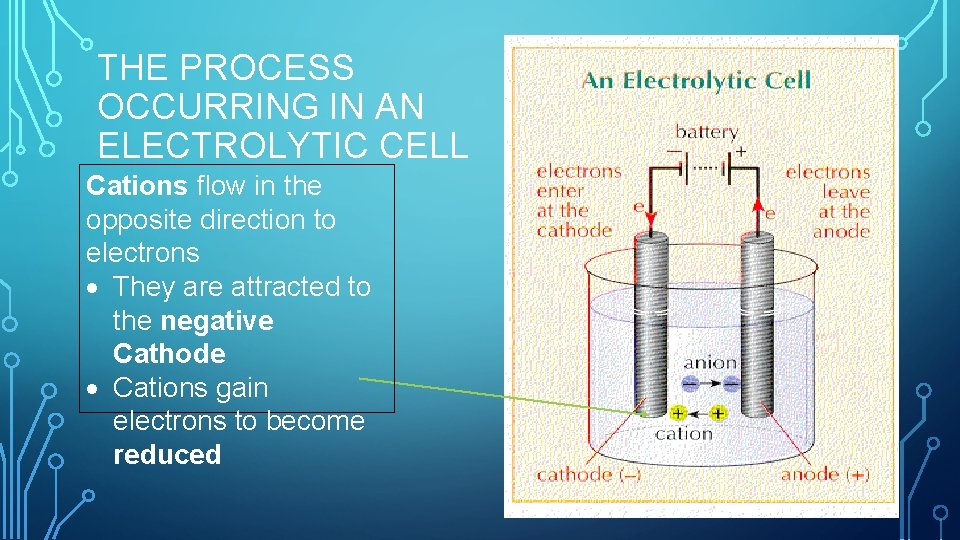
THE PROCESS OCCURRING IN AN ELECTROLYTIC CELL External Power Supply Source of electrons which can flow. (Current is carried in solution by ions).

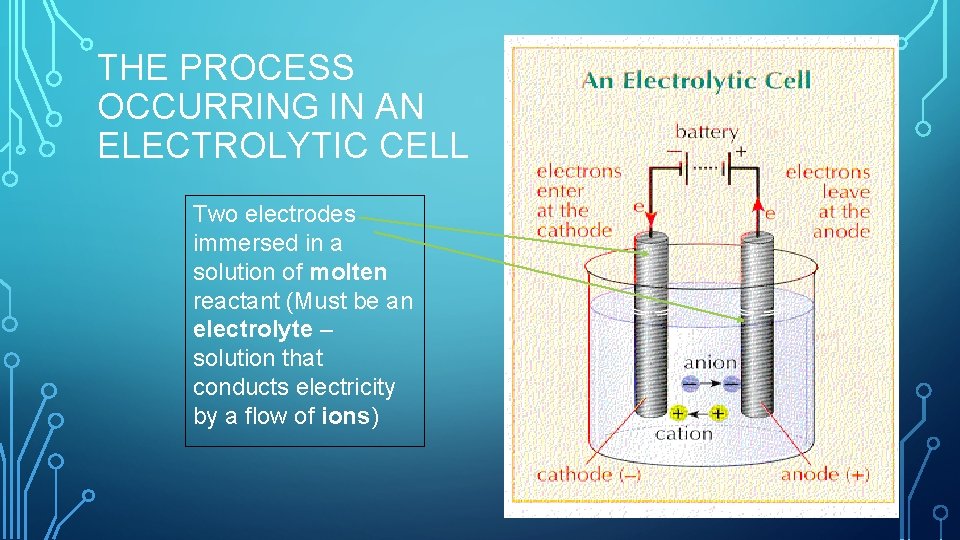
THE PROCESS OCCURRING IN AN ELECTROLYTIC CELL Two electrodes immersed in a solution of molten reactant (Must be an electrolyte – solution that conducts electricity by a flow of ions)

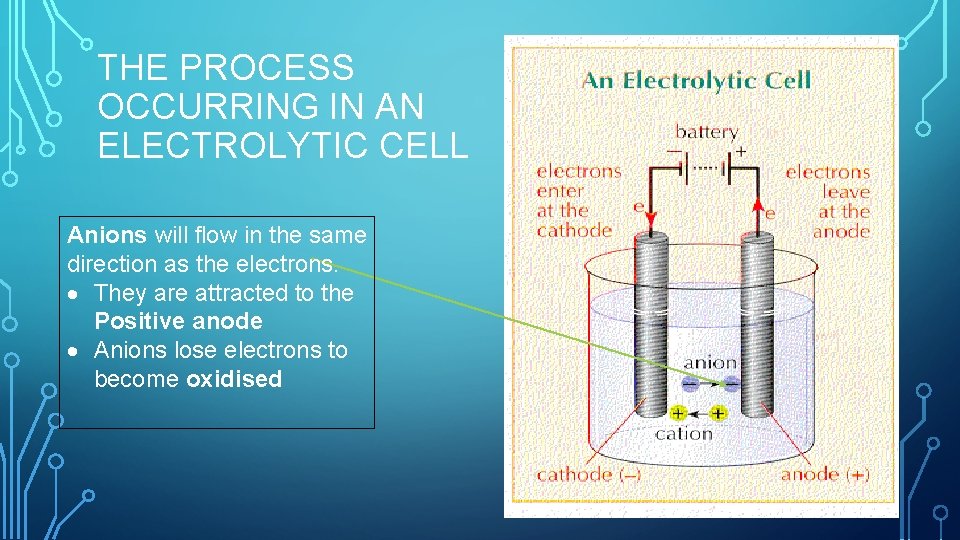
THE PROCESS OCCURRING IN AN ELECTROLYTIC CELL Anions will flow in the same direction as the electrons. They are attracted to the Positive anode Anions lose electrons to become oxidised

THE PROCESS OCCURRING IN AN ELECTROLYTIC CELL Cations flow in the opposite direction to electrons They are attracted to the negative Cathode Cations gain electrons to become reduced

ELECTROLYSIS ANIMATION • G: TGHSYear 13 Chemistry3. 7 redoxElectrolysis. docx

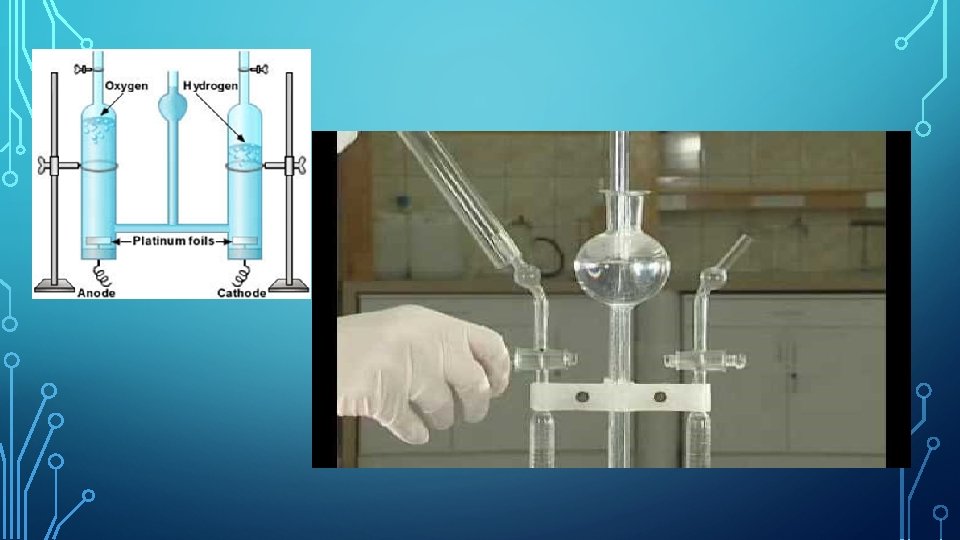
EXAMPLE – ELECTROLYSIS OF WATER Hoffman’s Voltameter is used for performing electrolysis of water and consists of platinum electrodes. Water is a molecular compound. Pure or distilled water is a non-electrolyte (it contains no charged particles that can carry a current). Therefore, a few crystals of an ionic compound (like sodium chloride – to produce ions) or a few drops of a strong acid (hydrochloric acid, sulphuric acid – they completely dissociate into ions) need to be added to the water to make it become an electrolyte. The anode and cathode are connected to a battery. The cell produces a small current of the order of a few milliamps and you will see bubbles appearing in the two arms of the voltameter. The anode collects oxygen (the anion is attracted to the positive anode) and the cathode arm collects hydrogen gas (the cation is attracted to the negative cathode)


solid state. EXTRACTION OF ALUMINIUM Aluminium ore is called bauxite. Bauxite contains aluminium oxide, water, iron oxide and other impurities. The purified dry ore, called alumina, is aluminium oxide Al 2 O 3. The alumina must be molten for electrolysis to work, since the ions are not free to move in the solid state

WRITE EQUATIONS FOR THE REACTIONS OCCURRING…. . The steel container is coated with carbon (graphite) and this is used as the negative electrode (cathode). Aluminium oxide (Al 2 O 3) is an ionic compound. When it is melted the Al 3+ and O 2 - ions are free to move and conduct electricity. Electrolysis of the alumina/cryolite solution produces aluminium at the negative cathode and oxygen at the positive anode.
