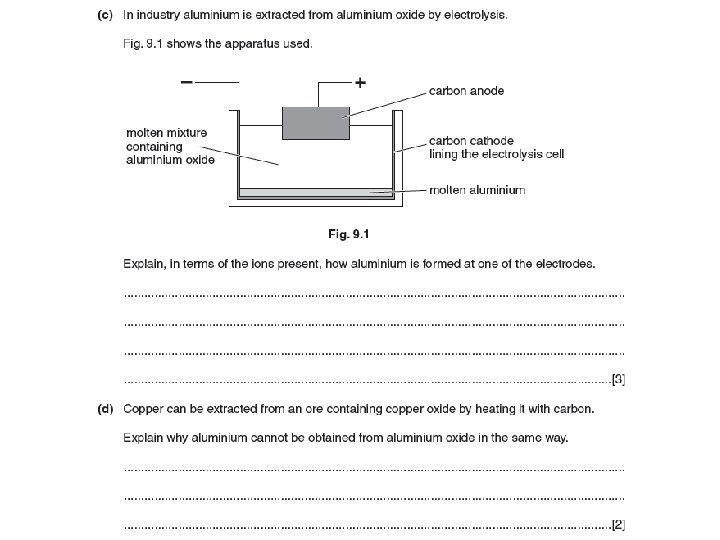
Electrolysis Electrolysis is the breakdown decomposition of a
Electrolysis ? ? ? Electrolysis is the break-down (decomposition) of a substance by electricity



• Electrolysis only happens in: - molten ionic liquids or - aqueous solutions containing ions. • There must be a complete circuit. • A lamp or ammeter shows that electricity is flowing around the circuit. • Requires electrical energy Electrode – (usually carbon) an electrical conductor which carries charge to or from a liquid undergoing electrolysis. Electrolyte - a molten (heat required) or aqueous solution through which an electrical current can flow. Salt
Electrolysis of copper chloride Electrode Anode (+ve) Cathode (-ve) Observation Bubbles seen; smells like a swimming pool; bleaches litmus paper A pink solid forms on the electrode; black ppt is formed Deduction Chlorine gas is being given off Copper solid is being deposited onto the electrode
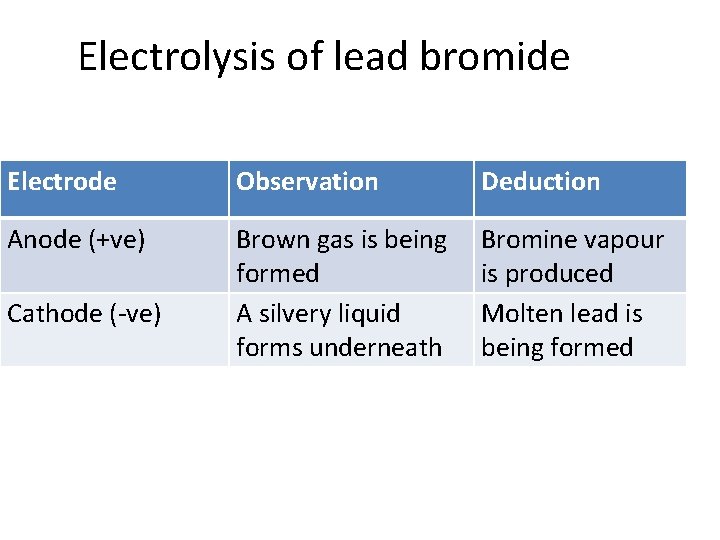
Electrolysis of lead bromide Electrode Observation Deduction Anode (+ve) Brown gas is being formed A silvery liquid forms underneath Bromine vapour is produced Molten lead is being formed Cathode (-ve)
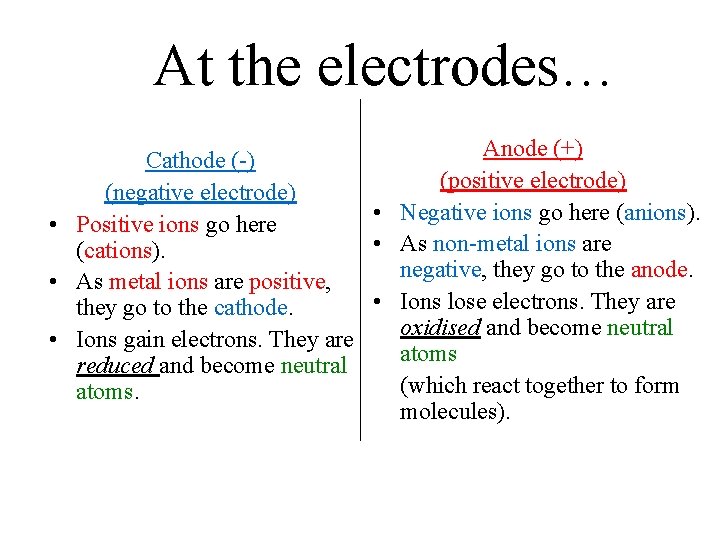
At the electrodes… Anode (+) Cathode (-) (positive electrode) (negative electrode) • Negative ions go here (anions). • Positive ions go here • As non-metal ions are (cations). negative, they go to the anode. • As metal ions are positive, • Ions lose electrons. They are they go to the cathode. oxidised and become neutral • Ions gain electrons. They are atoms reduced and become neutral (which react together to form atoms. molecules).

Oxidation Is Loss Reduction Is OILRIG Gain (of electrons) Oxidised or Oxidation is the gain of oxygen Reduced or Reduction is loss of oxygen
- Slides: 10