Electrolysis Chemical reaction caused by the passage of
Electrolysis • Chemical reaction caused by the passage of an electric current through a liquid known as the electrolyte

Definitions • Electrolyte - liquid in which electrolysis takes place. Usually an ionic solution but it can also be a fused [melted] ionic compound • • • Anode - positive electrode. Positive because the battery sucks electrons out of it • Cation - positive ion. Called cation because it is attracted to the opposite charge of the cathode. • Inert Electrodes - do not react with the electrolyte Graphite and Pt • Active electrodes - react with electrolyte e. g. Copper and iron Cathode. Negative electrode. Negative because the battery pumps electrons into it. Anion - negative ion. Called anion because it is attracted to the opposite charge of the anode

Electrolysis of Potassium Iodide • Solution of KI + phenolphthalein • Brown I 2(s) forms at the positive electrode and some yellow/orange I 3 - forms in solution. At the negative electrode, H+ is again reduced to H 2(g) and the phenolphthalein turns pink due to the OH- ions.

Electrolysis of Potassium Iodide
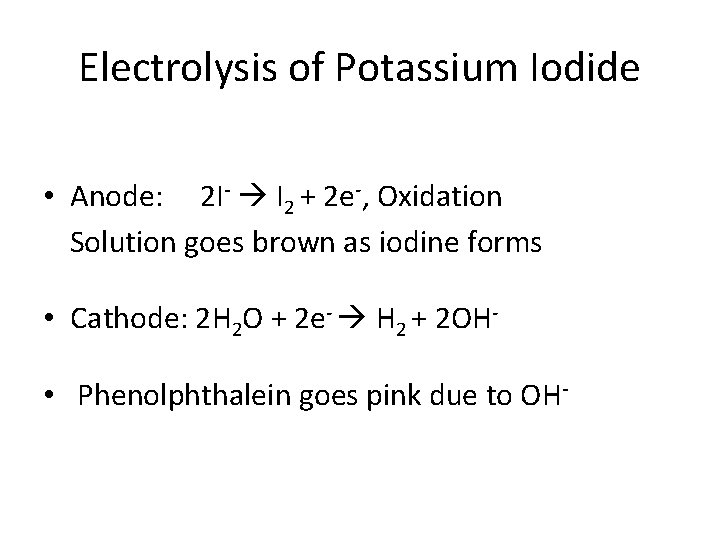
Electrolysis of Potassium Iodide • Anode: 2 I- I 2 + 2 e-, Oxidation Solution goes brown as iodine forms • Cathode: 2 H 2 O + 2 e- H 2 + 2 OH • Phenolphthalein goes pink due to OH-
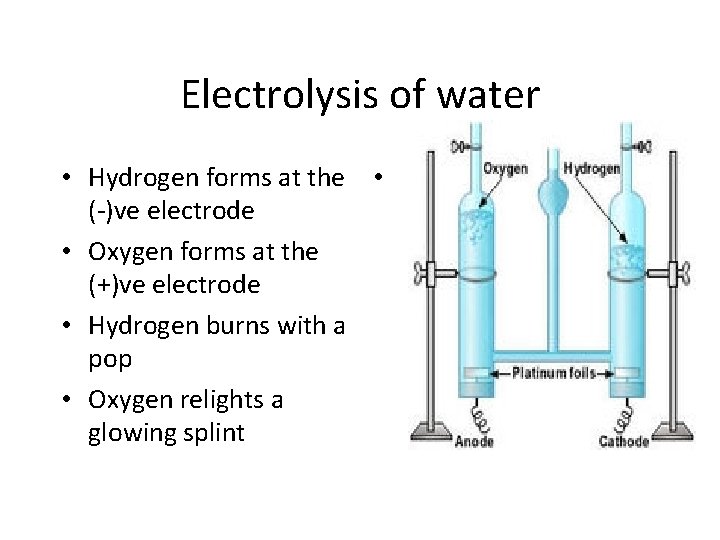
Electrolysis of water • Hydrogen forms at the • (-)ve electrode • Oxygen forms at the (+)ve electrode • Hydrogen burns with a pop • Oxygen relights a glowing splint
Electrolysis of Acidified Water using Hoffman Voltameter Water is oxidized at the anode (negative) and reduced at the cathode (positive). Cathode: 2 H+ + 2 e- --> H 2 Anode: 2 H 2 O --> O 2 + 4 H+ + 4 e- (divide by 2 if asked for both)

Sodium Sulphate and Universal Indicator
Electrolysis of Sodium sulphate • Solution of Na 2 SO 4 + universal indicator • H+ ions are produced at the positive electrode (oxidation of O 2 - in water) while OH- ions are produced at the negative electrode as the H+ in water is reduced to H 2(g).
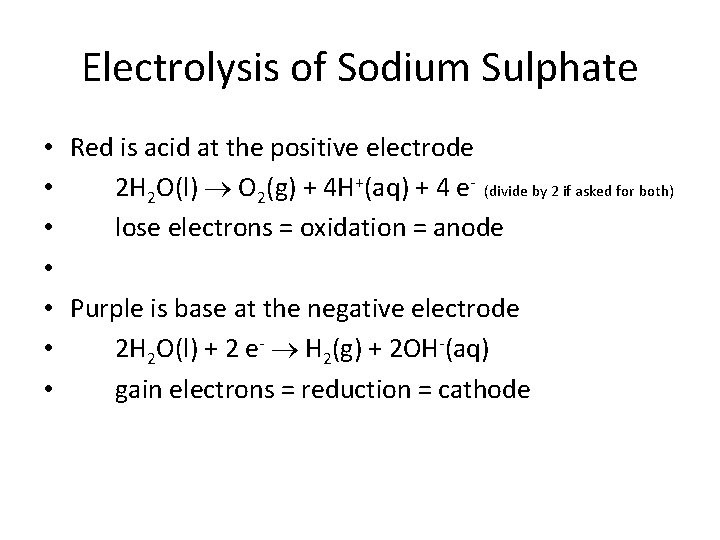
Electrolysis of Sodium Sulphate • Red is acid at the positive electrode • 2 H 2 O(l) O 2(g) + 4 H+(aq) + 4 e- (divide by 2 if asked for both) • lose electrons = oxidation = anode • • Purple is base at the negative electrode • 2 H 2 O(l) + 2 e- H 2(g) + 2 OH-(aq) • gain electrons = reduction = cathode
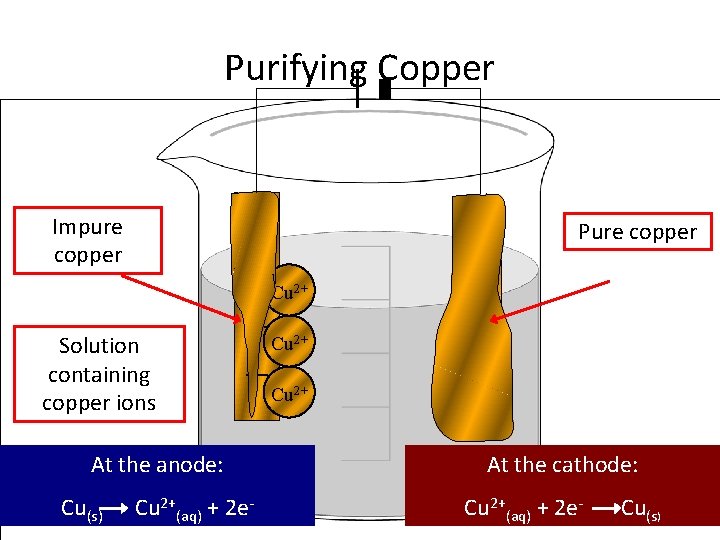
Purifying Copper Impure copper Solution containing copper ions + + Cu At the anode: Cu 10/02/2022 (s) Cu 2+(aq) + 2 e- 2+ --- Pure copper 2+ 2+ At the cathode: Cu 2+(aq) + 2 e- Cu(s)
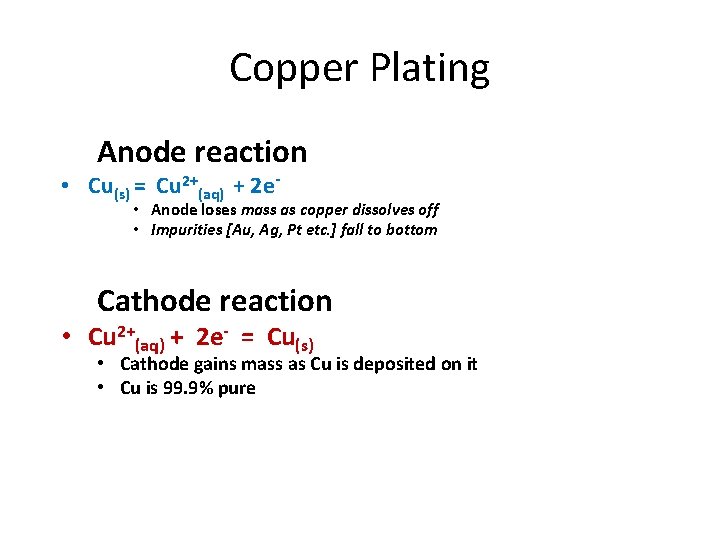
Copper Plating Anode reaction • Cu(s) = Cu 2+(aq) + 2 e- • Anode loses mass as copper dissolves off • Impurities [Au, Ag, Pt etc. ] fall to bottom Cathode reaction • Cu 2+(aq) + 2 e- = Cu(s) • Cathode gains mass as Cu is deposited on it • Cu is 99. 9% pure
- Slides: 12