Electrolysis background Electrodes Conducting liquid electrolyte Electrolysis is
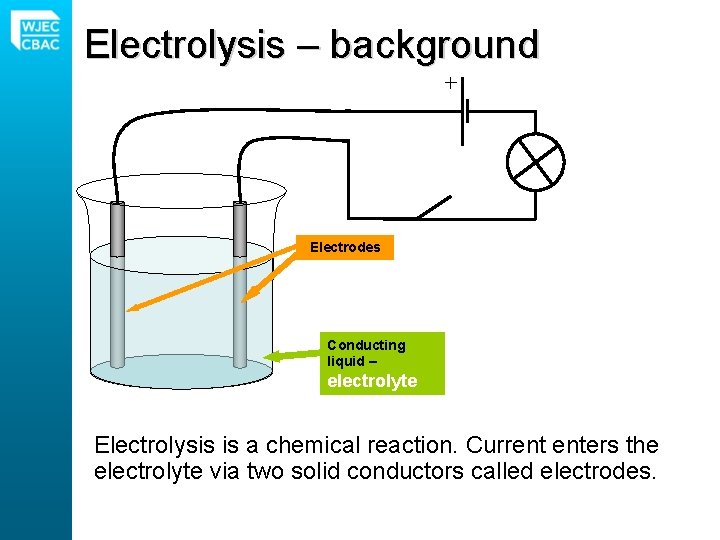
Electrolysis – background Electrodes Conducting liquid – electrolyte Electrolysis is a chemical reaction. Current enters the electrolyte via two solid conductors called electrodes.
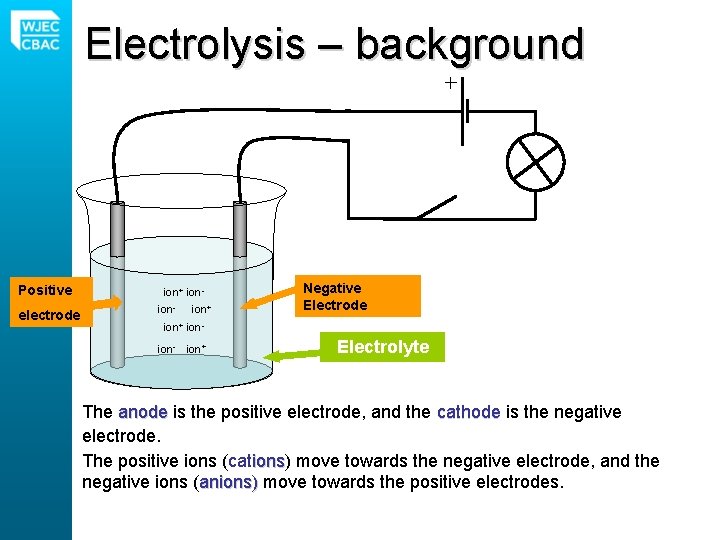
Electrolysis – background Positive electrode ion+ ionion- ion+ Negative Electrode Electrolyte The anode is the positive electrode, and the cathode is the negative electrode. The positive ions (cations) ions move towards the negative electrode, and the negative ions (anions) move towards the positive electrodes.
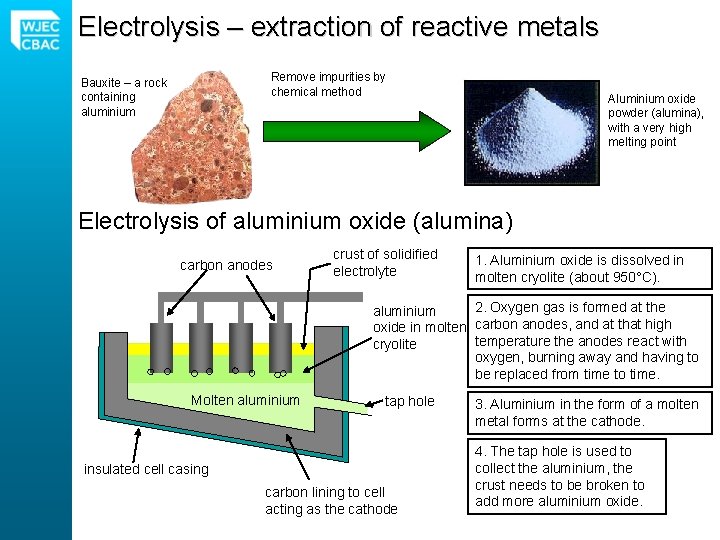
Electrolysis – extraction of reactive metals Remove impurities by chemical method Bauxite – a rock containing aluminium Aluminium oxide powder (alumina), with a very high melting point Electrolysis of aluminium oxide (alumina) carbon anodes crust of solidified electrolyte 1. Aluminium oxide is dissolved in molten cryolite (about 950°C). 2. Oxygen gas is formed at the aluminium oxide in molten carbon anodes, and at that high temperature the anodes react with cryolite oxygen, burning away and having to be replaced from time to time. Molten aluminium tap hole insulated cell casing carbon lining to cell acting as the cathode 3. Aluminium in the form of a molten metal forms at the cathode. 4. The tap hole is used to collect the aluminium, the crust needs to be broken to add more aluminium oxide.
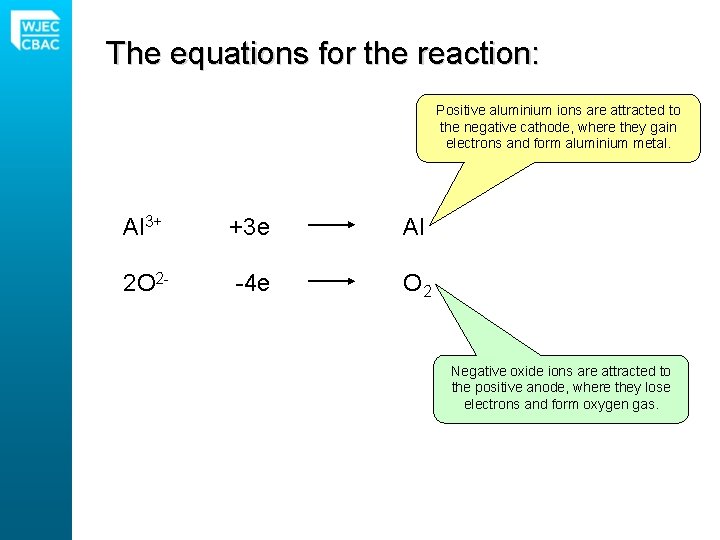
The equations for the reaction: Positive aluminium ions are attracted to the negative cathode, where they gain electrons and form aluminium metal. Al 3+ +3 e Al 2 O 2 - -4 e O 2 Negative oxide ions are attracted to the positive anode, where they lose electrons and form oxygen gas.
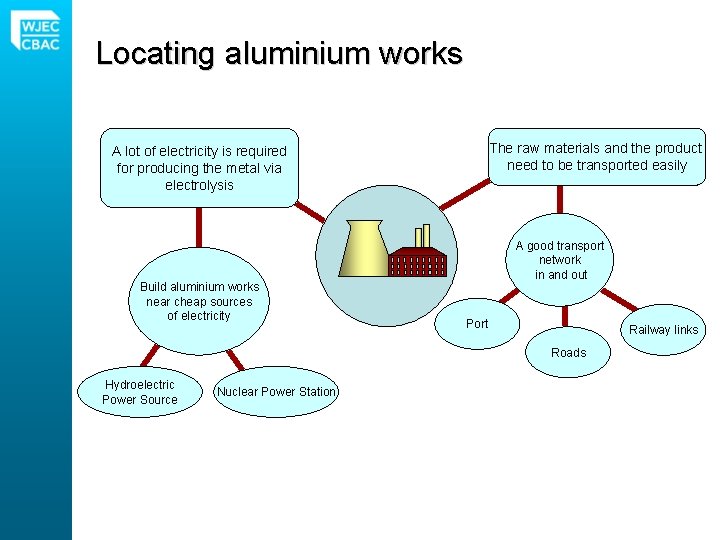
Locating aluminium works The raw materials and the product need to be transported easily A lot of electricity is required for producing the metal via electrolysis Build aluminium works near cheap sources of electricity A good transport network in and out Port Railway links Roads Hydroelectric Power Source Nuclear Power Station
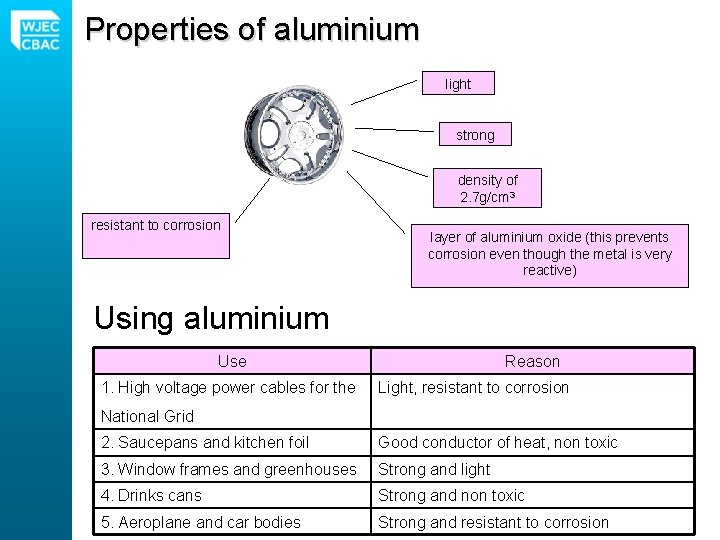
Properties of aluminium light strong density of 2. 7 g/cm 3 resistant to corrosion layer of aluminium oxide (this prevents corrosion even though the metal is very reactive) Using aluminium Use 1. High voltage power cables for the Reason Light, resistant to corrosion National Grid 2. Saucepans and kitchen foil Good conductor of heat, non toxic 3. Window frames and greenhouses Strong and light 4. Drinks cans Strong and non toxic 5. Aeroplane and car bodies Strong and resistant to corrosion
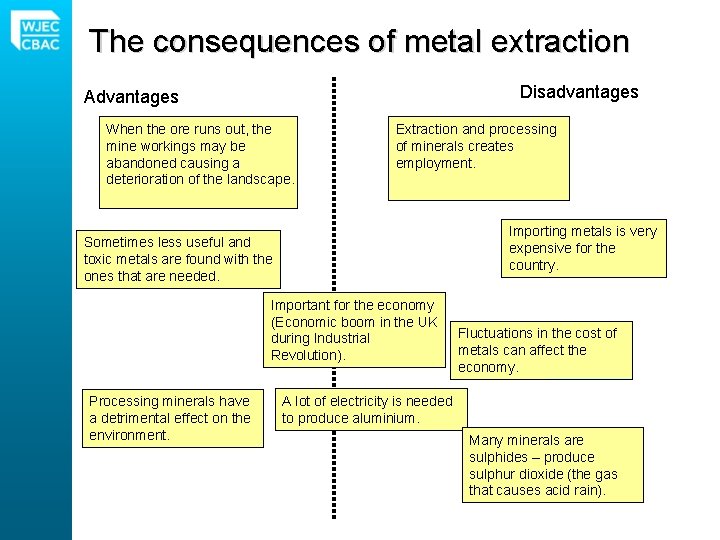
The consequences of metal extraction Disadvantages Advantages When the ore runs out, the mine workings may be abandoned causing a deterioration of the landscape. Extraction and processing of minerals creates employment. Importing metals is very expensive for the country. Sometimes less useful and toxic metals are found with the ones that are needed. Important for the economy (Economic boom in the UK during Industrial Revolution). Processing minerals have a detrimental effect on the environment. Fluctuations in the cost of metals can affect the economy. A lot of electricity is needed to produce aluminium. Many minerals are sulphides – produce sulphur dioxide (the gas that causes acid rain).
- Slides: 7