ELECTROCHEMISTRY WHAT IS ELECTROCHEMISTRY The branch of chemistry
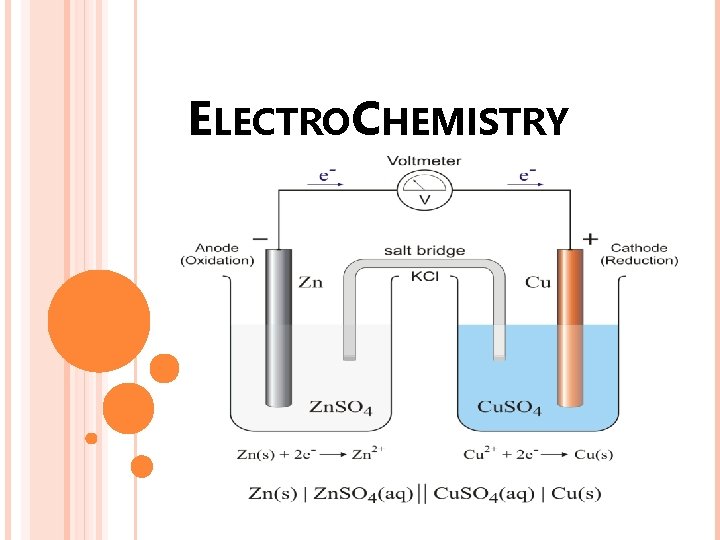
ELECTROCHEMISTRY
WHAT IS ELECTROCHEMISTRY? The branch of chemistry that examines the phenomena resulting from combined chemical and electrical effects. Electricity can be generated by movements of electrons from one element to another - a redox reaction, or oxidation-reduction reaction.
TYPES OF PROCESSES: Electrolytic processes: � Non-spontaneous reactions in which chemical changes occur caused by the application of an electrical current Galvanic or Voltaic processes: � Spontaneous chemical reactions that result in the production of electrical energy

REDOX REACTIONS

REDOX REVIEW: Redox Reaction – rxn in which one species is oxidized another is reduced Oxidation - Rxn in which e- are lost Reduction - Rxn in which e- are gained Oxidizing agent (AKA oxidant) – Substance which enables another to be oxidized The RA contributes e- from another substance Reducing Agent (AKA reductant) – Substance which enables another to be reduced The RA contributes e- to another substance

STEPS FOR BALANCING REDOX RXNS: Process for Balancing Redox Eqns in Acidic Sol – See Unit 6 IChem Notes Process for Balancing Redox Eqns in Basic Sol. Assign ox numbers ID substance which is oxidized For substance oxidized: � Write half-rxn � Balance atoms excluding H and O � Balance O by adding H 2 O � Balance H by adding H+ � Balance charge by adding e. Repeat “c” for substance reduced Multiply half-rxns by integers so that number of e- in each half-rxn is the same Add the half-rxns together Convert H+ to H 2 O by adding OH- to both sides. Simplify substances appearing on both sides of eqn Check number of each atom and charge

GALVANIC CELLS

VOLTAIC CELLS (AKA GALVANIC CELLS) Device in which a spontaneous redox occurs with the transfer of electrons flowing through an external circuit Voltaic cells consists of two half-cells. Oxidation Half-Cell Reduction Half-Cell Voltaic cells also consist of a salt bridge which keeps the cell electrically neutral.
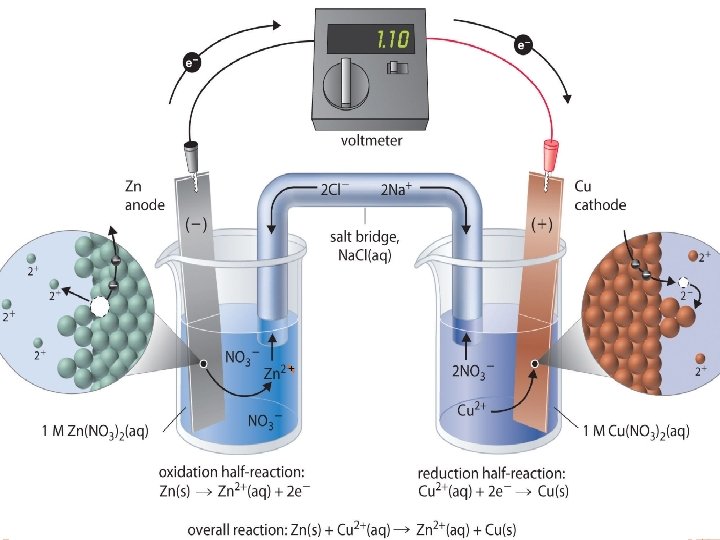

Oxidation Half-Cell in which the oxidation occurs A piece of the solid which will oxidize is placed in a solution of its ions The piece of solid is known as the anode At the anode the oxidation rxn occurs: � Metal atoms from the anode release electrons into the anode and go into solution as ions. Over time, the mass of the anode decreases as the solid atoms lose electrons and go into solution as cations.

Reduction Half-Cell in which the reduction occurs A piece of the solid metal which will reduce in the redox rxn is placed in a solution of its ions The piece of solid is known as the cathode At the cathode the reduction rxn occurs: Metal ions gain electrons from the cathode and deposit as solids on the cathode The salt bridge allows both cells to remain electrically neutral. Anions flow from the salt bridge into the oxidation half-cell. Cations flow from the salt bridge into the reduction half-cell.

Salt Bridge Connection between the half-cells containing an ionic paste. Purpose: allows both cells to remain electrically neutral. Anions from the salt bridge flow into the oxidation half-cell. Cations from the salt bridge flow into the reduction half-cell.

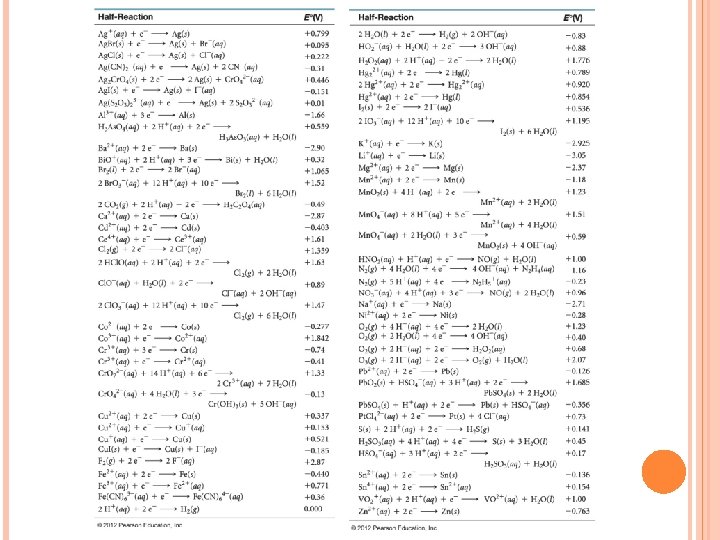
Determining ½ cells in a Galvanic Cell The standard reduction potential(E˚red) measures the relative likelihood of reduction. (see table on next slide. ) The higher the value, the more likely that a species will reduce. In a Galvanic cell, the ½ rxn with the higher E˚red will be the reduction ½ cell. The ½ rxn with the lower E ˚red will be the oxidation ½ cell. Consider the Zn/Zn 2+ Cu. Cu 2+ Galvanic cell from the picture above. The E˚red values are: -0. 763 V Cu 2+ (aq) + 2 e- →Cu (s) + 0. 337 V Since Cu has the higher E˚red , it will be the cathode. The ½ reduction cell will be Cu 2+(aq) + 2 e →Cu(s) Since Zn has the lower E ˚red , it will be the anode. The ½ oxidation cell will be Zn(s) → 2 e + Zn 2+(aq) + 2 e- →Zn(s)
- Slides: 14