Electrochemistry V Cell Potential Electrical Work Free Energy










- Slides: 12

Electrochemistry V Cell Potential, Electrical Work & Free Energy

Work The work that is accomplished is due to the push behind the electron flow. The driving force, emf, is defined in terms of potential difference in volts between 2 points in the circuit. 1 volt = 1 Joule of work/ Coulomb of charge transferred

Work is viewed from the point of view of the system. Thus, work flowing out of the system is indicated by a negative (-) sign. Therefore, cell potential and work have opposite signs. E = -w/q or work/charge -w= E q

Maximum work comes from maximum potential. -wmax = q Emax or wmax=-q Emax However during any electrical work, current must flow and from the flow some energy is lost through friction creating heat. Thus, actual work is always less than the calculated maximum.

Entropy As electrical energy is lost to heat energy, the wires get hot and entropy increases.

Cell Efficiency Suppose you have a galvanic cell. Your cell has a maximum potential (at zero current) of 2. 50 v. In one experiment 1. 33 moles of electrons were passed through the cell at an average potential of 2. 10 v Actual work done w=-q E E = actual potential of current flow 2. 10 v

Cell Efficiency q = charge in coulombs 1 mole electrons = 1 Faraday = 96, 485 q =n. F =(1. 33 mole e-)(96, 485 C/mol e-) w=-q E =-(1. 33 mol e-)(96485 c/mol e-)(2. 10 J/C) = -2. 69 x 105 J

Cell Efficiency The max would be Wmax = -q E =-(1. 33 mol e-)(96485 C/mol e-)(2. 50 J/C) = -3. 21 x 105 J Cell Efficiency w/wmax x 100 -2. 69 x 105 J X 100 = 83. 8% -3. 21 x 105 J

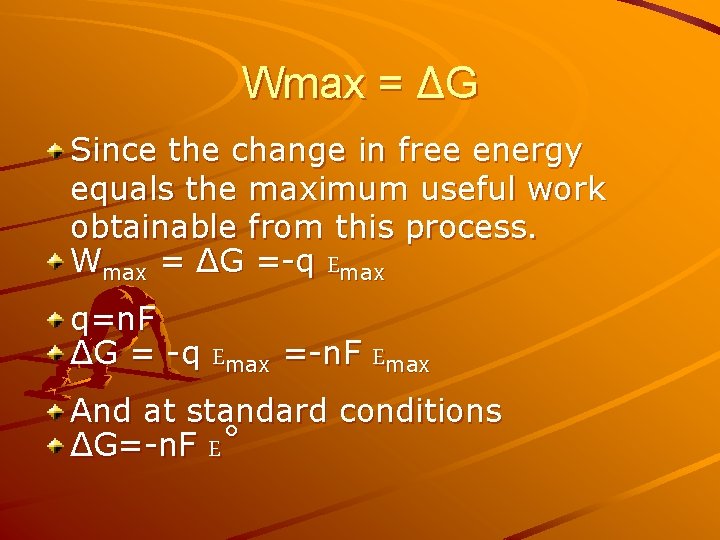
Wmax = ΔG Since the change in free energy equals the maximum useful work obtainable from this process. Wmax = ΔG =-q Emax q=n. F ΔG = -q Emax =-n. F Emax And at standard conditions ΔG=-n. F E˚

Wmax = ΔG So that the maximum cell potential is directly related to the free energy difference between the products and reactants in the cell. This also confirms that the spontaneous galvanic cell has a positive Ecell which gives a - ΔG

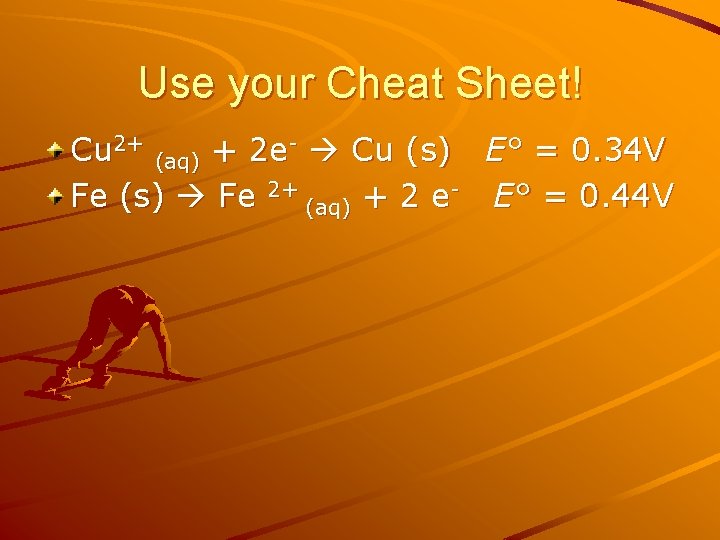
Using Standard reduction potential Calculate ΔG˚ for the reaction Cu 2+(aq)+ Fe(s) Cu(s) + Fe 2+(aq) Is this reaction spontaneous?

Use your Cheat Sheet! Cu 2+ (aq) + 2 e- Cu (s) E° = 0. 34 V Fe (s) Fe 2+ (aq) + 2 e- E° = 0. 44 V