Electrochemistry Review Which is a very strong reducing





- Slides: 15

Electrochemistry Review

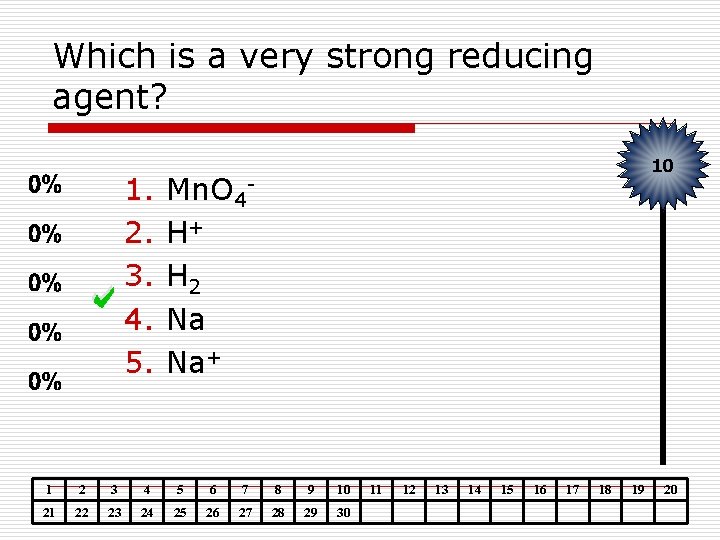
Which is a very strong reducing agent? 1. 2. 3. 4. 5. 10 Mn. O 4 H+ H 2 Na Na+ 1 2 3 4 5 6 7 8 9 10 21 22 23 24 25 26 27 28 29 30 11 12 13 14 15 16 17 18 19 20

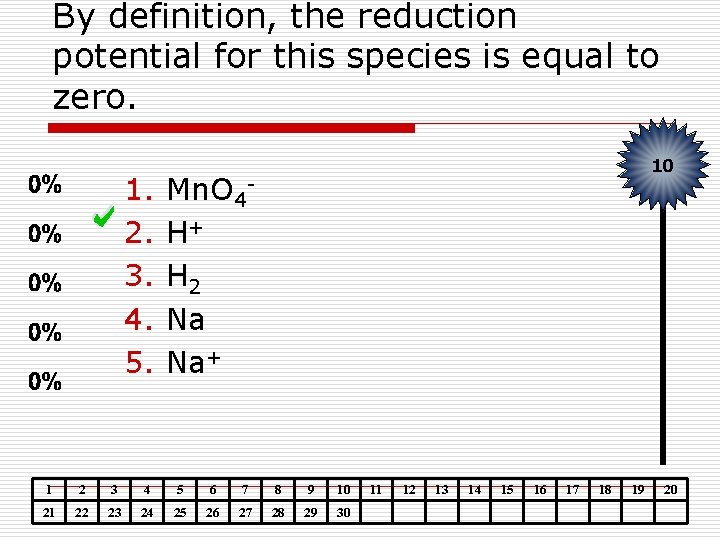
By definition, the reduction potential for this species is equal to zero. 1. 2. 3. 4. 5. 10 Mn. O 4 H+ H 2 Na Na+ 1 2 3 4 5 6 7 8 9 10 21 22 23 24 25 26 27 28 29 30 11 12 13 14 15 16 17 18 19 20

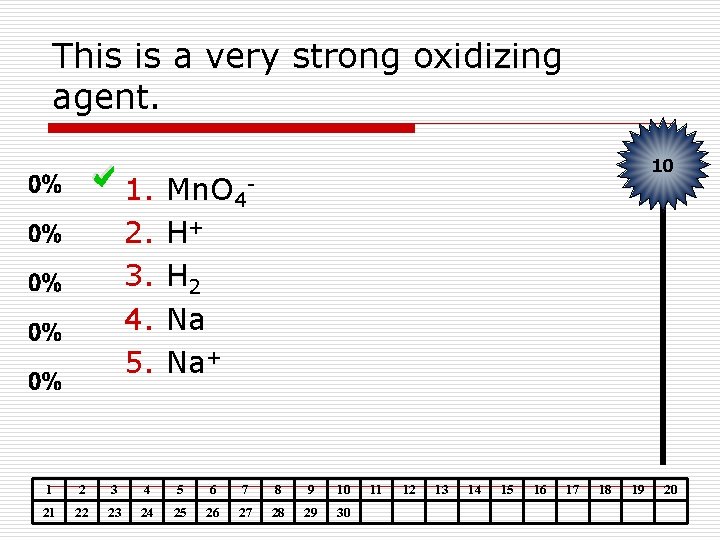
This is a very strong oxidizing agent. 1. 2. 3. 4. 5. 10 Mn. O 4 H+ H 2 Na Na+ 1 2 3 4 5 6 7 8 9 10 21 22 23 24 25 26 27 28 29 30 11 12 13 14 15 16 17 18 19 20

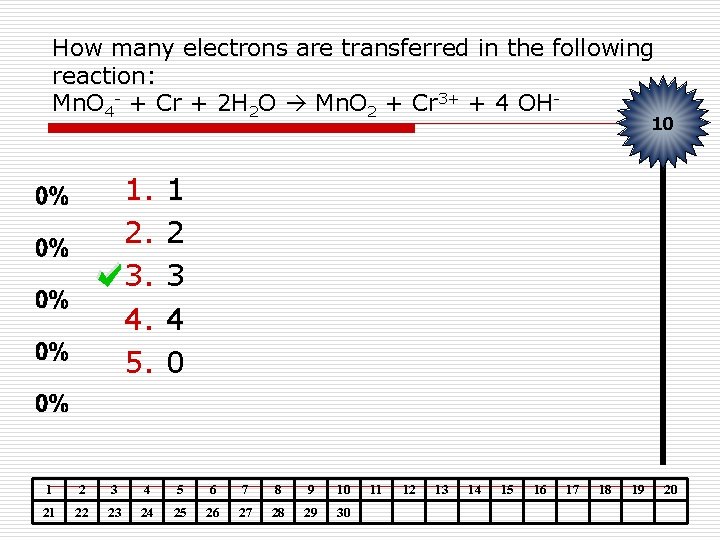
How many electrons are transferred in the following reaction: Mn. O 4 - + Cr + 2 H 2 O Mn. O 2 + Cr 3+ + 4 OH- 10 1. 2. 3. 4. 5. 1 2 3 4 0 1 2 3 4 5 6 7 8 9 10 21 22 23 24 25 26 27 28 29 30 11 12 13 14 15 16 17 18 19 20

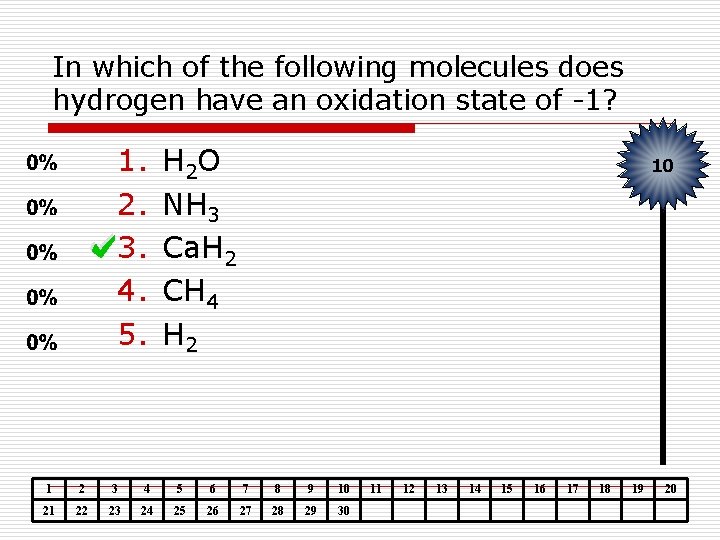
In which of the following molecules does hydrogen have an oxidation state of -1? 1. 2. 3. 4. 5. H 2 O NH 3 Ca. H 2 CH 4 H 2 10 1 2 3 4 5 6 7 8 9 10 21 22 23 24 25 26 27 28 29 30 11 12 13 14 15 16 17 18 19 20

When solid copper shavings are placed in a solution of dilute HNO 3, Cu 2+ ions appear and NO gas bubbles form. Which of the following has occurred? 1. 2. 3. 4. 5. Cu has been oxidized by H+ Cu has been oxidized by NO 3 Cu has been reduced by NO 3 - has been oxidized by H+ NO 3 - has been reduced by H+ 1 2 3 4 5 6 7 8 9 10 21 22 23 24 25 26 27 28 29 30 11 12 13 14 15 16 17 18 10 19 20

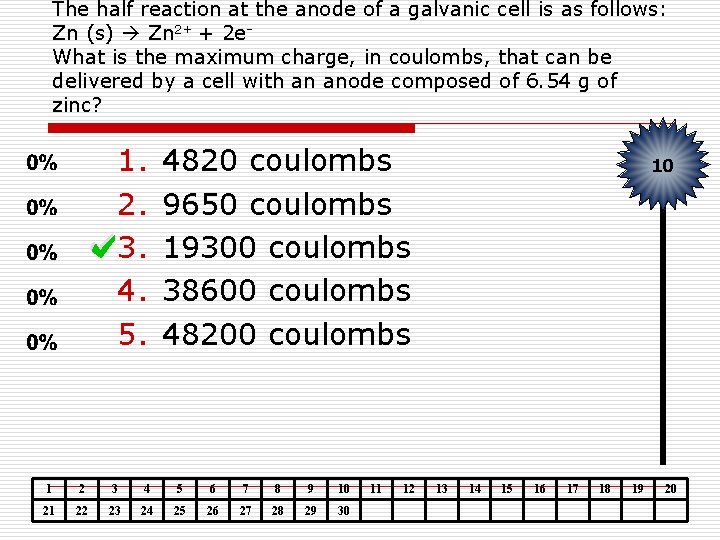
The half reaction at the anode of a galvanic cell is as follows: Zn (s) Zn 2+ + 2 e. What is the maximum charge, in coulombs, that can be delivered by a cell with an anode composed of 6. 54 g of zinc? 1. 2. 3. 4. 5. 4820 coulombs 9650 coulombs 19300 coulombs 38600 coulombs 48200 coulombs 1 2 3 4 5 6 7 8 9 10 21 22 23 24 25 26 27 28 29 30 11 12 10 13 14 15 16 17 18 19 20

Ni(s) + Cu 2+ Ni 2+ + Cu(s) The reaction above takes place in a galvanic cell. If the initial concentration of Ni 2+ is increased while the initial concentration of Cu 2+ remains the same, what will be the effect on Q and E? 1. Q and E will increase 2. Q and E will decrease 3. Q will increase and E will decrease 4. Q will decrease and E will increase 5. Q and E will not change 1 2 3 4 5 6 7 8 9 10 21 22 23 24 25 26 27 28 29 30 11 12 13 14 15 16 10 17 18 19 20

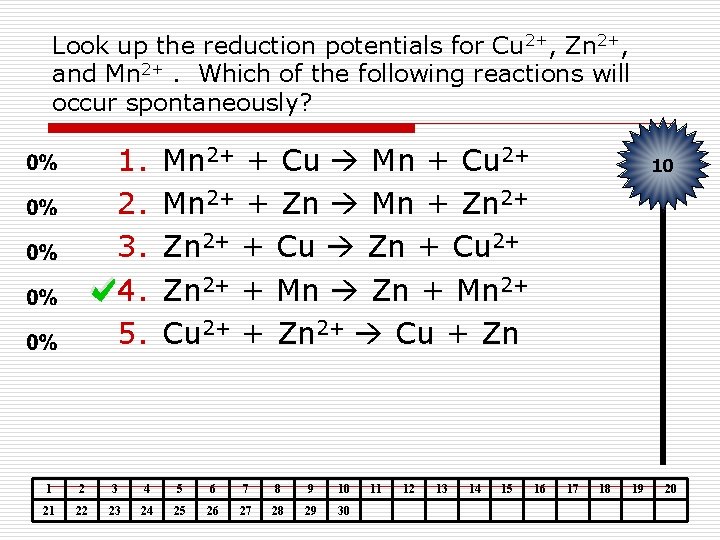
Look up the reduction potentials for Cu 2+, Zn 2+, and Mn 2+. Which of the following reactions will occur spontaneously? 1. 2. 3. 4. 5. Mn 2+ + Cu Mn + Cu 2+ Mn 2+ + Zn Mn + Zn 2+ + Cu Zn + Cu 2+ Zn 2+ + Mn Zn + Mn 2+ Cu 2+ + Zn 2+ Cu + Zn 1 2 3 4 5 6 7 8 9 10 21 22 23 24 25 26 27 28 29 30 11 12 13 14 15 10 16 17 18 19 20

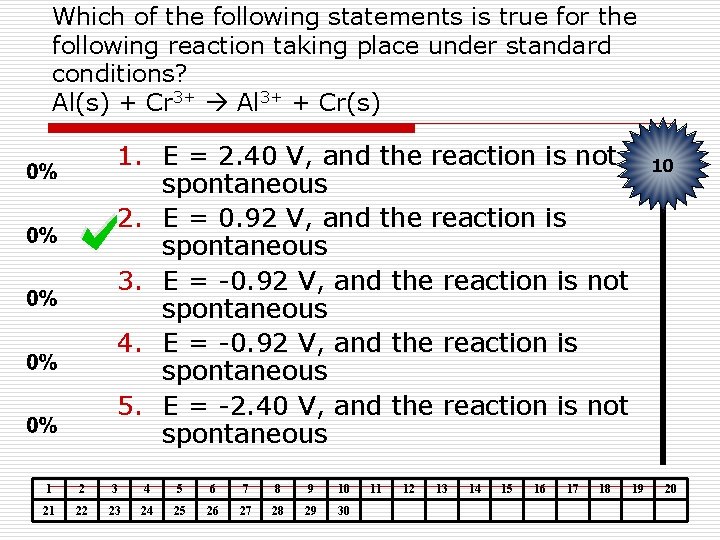
Which of the following statements is true for the following reaction taking place under standard conditions? Al(s) + Cr 3+ Al 3+ + Cr(s) 1. E = 2. 40 V, and the reaction is not spontaneous 2. E = 0. 92 V, and the reaction is spontaneous 3. E = -0. 92 V, and the reaction is not spontaneous 4. E = -0. 92 V, and the reaction is spontaneous 5. E = -2. 40 V, and the reaction is not spontaneous 1 2 3 4 5 6 7 8 9 10 21 22 23 24 25 26 27 28 29 30 11 12 13 14 15 16 17 18 10 19 20

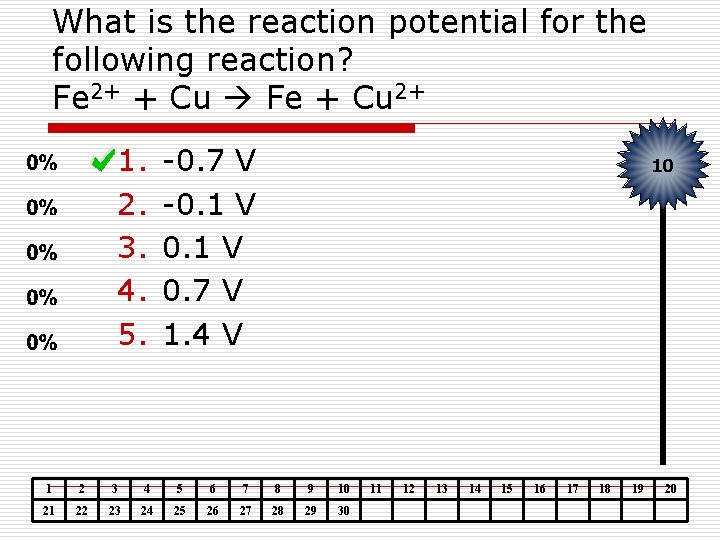
What is the reaction potential for the following reaction? Fe 2+ + Cu Fe + Cu 2+ 1. 2. 3. 4. 5. -0. 7 V -0. 1 V 0. 7 V 1. 4 V 10 1 2 3 4 5 6 7 8 9 10 21 22 23 24 25 26 27 28 29 30 11 12 13 14 15 16 17 18 19 20

An electrochemical cell was created by placing a zinc electrode in a 1. 00 M solution of Zn. SO 4 and placing a copper electrode in a 1. 00 M solution of Cu. SO 4. The two compartments are connected by a salt bridge, and the following reaction occurred at 25 o. C. Zn(s) + Cu 2+ Zn 2+ + Cu(s) o o What is the standard potential for the cell? How much work can the cell do? What is the value of Keq for the reaction? At a certain point in the progress of the reaction, [Cu 2+] drops to 0. 10 M and [Zn 2+] increases to 1. 90 M. What is the cell potential at this point?

An 800 m. L sample of 0. 800 M Ag+ solution was electrolyzed, resulting in the formation of solid silver and oxygen gas. The solution was subjected to a current of 2. 00 A for 10. 0 minutes. The solution became progressively more acidic as the reaction progressed. o Write the two half-reactions that occur, stating which takes place at the anode and which the cathode. o If the oxygen gas produced in the electrolysis was collected at STP, what was its volume? o What was the mass of solid silver produced in the electrolysis? o If the solution was neutral at the start of the electrolysis, what was the p. H of the solution when the process was complete?

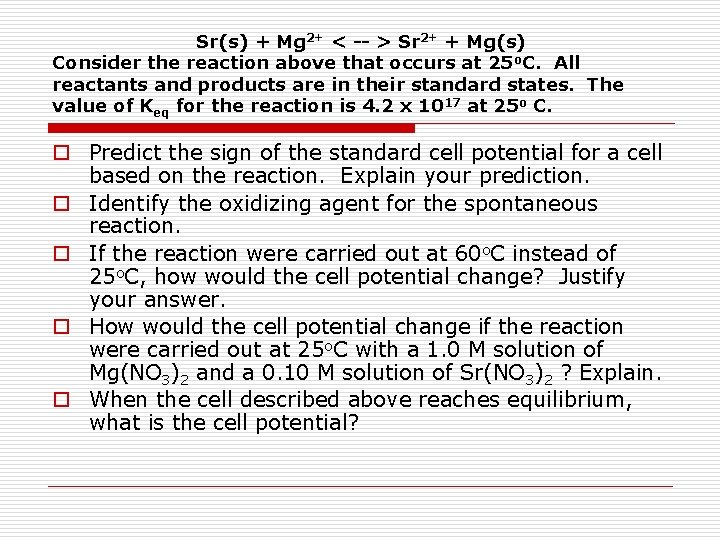
Sr(s) + Mg 2+ < -- > Sr 2+ + Mg(s) Consider the reaction above that occurs at 25 o. C. All reactants and products are in their standard states. The value of Keq for the reaction is 4. 2 x 1017 at 25 o C. o Predict the sign of the standard cell potential for a cell based on the reaction. Explain your prediction. o Identify the oxidizing agent for the spontaneous reaction. o If the reaction were carried out at 60 o. C instead of 25 o. C, how would the cell potential change? Justify your answer. o How would the cell potential change if the reaction were carried out at 25 o. C with a 1. 0 M solution of Mg(NO 3)2 and a 0. 10 M solution of Sr(NO 3)2 ? Explain. o When the cell described above reaches equilibrium, what is the cell potential?