Electrochemistry Putting redox reactions to work Redox review











![Concentration Cells Ecell = 2. 71 V – {(0. 0592/2)(log([0. 75]/[0. 5]))} Ecell = Concentration Cells Ecell = 2. 71 V – {(0. 0592/2)(log([0. 75]/[0. 5]))} Ecell =](https://slidetodoc.com/presentation_image_h2/25c1116fee6fe3b9ffb0f3ddabaec40a/image-25.jpg)

- Slides: 26

Electrochemistry Putting redox reactions to work

Redox review Electrons are transferred Lose Electrons Oxidation Gain Electrons Reduction

Electrochemical Cells Made of two half-cells Based upon two half-reactions Electrons travel between the two half-cells

Galvanic Cells Also called voltaic cells Convert chemical energy into electrical energy Spontaneous

Electrolytic Cells Convert electrical energy into chemical energy Non-spontaneous

Making a Galvanic Cell Write the reaction for solid magnesium placed in a copper (II) sulfate solution. Mg (s) + Cu. SO 4 (aq) Mg. SO 4 (aq) + Cu (s)

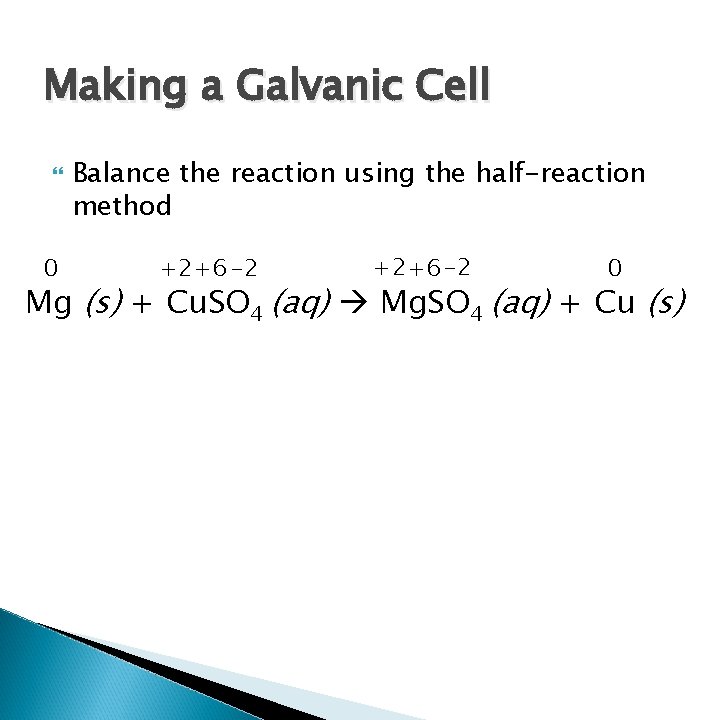
Making a Galvanic Cell 0 Balance the reaction using the half-reaction method +2+6 -2 0 Mg (s) + Cu. SO 4 (aq) Mg. SO 4 (aq) + Cu (s)

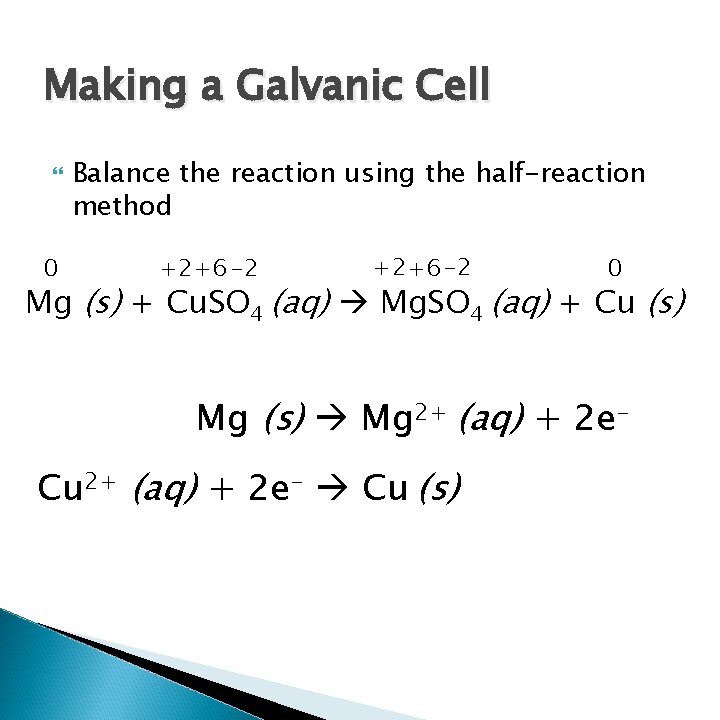
Making a Galvanic Cell 0 Balance the reaction using the half-reaction method +2+6 -2 0 Mg (s) + Cu. SO 4 (aq) Mg. SO 4 (aq) + Cu (s) Mg (s) Mg 2+ (aq) + 2 e. Cu 2+ (aq) + 2 e- Cu (s)

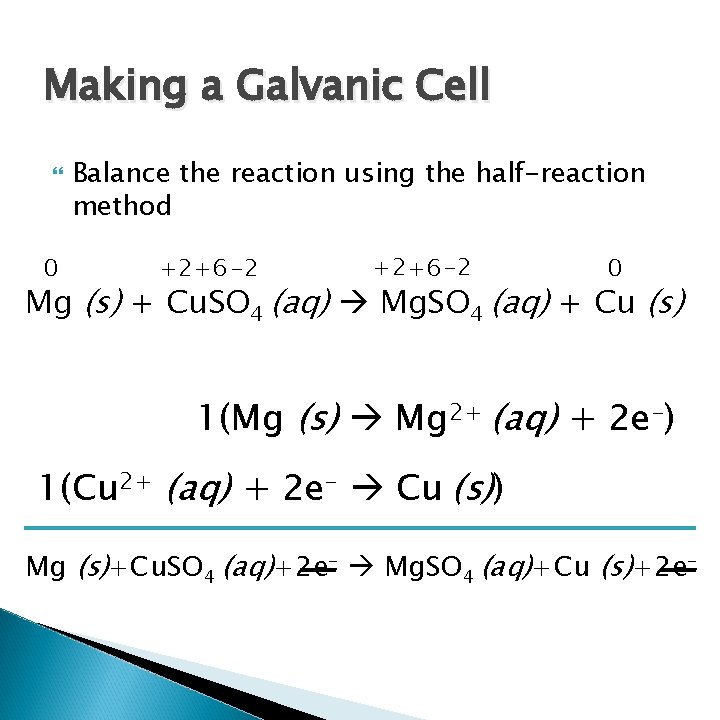
Making a Galvanic Cell 0 Balance the reaction using the half-reaction method +2+6 -2 0 Mg (s) + Cu. SO 4 (aq) Mg. SO 4 (aq) + Cu (s) 1(Mg (s) Mg 2+ (aq) + 2 e-) 1(Cu 2+ (aq) + 2 e- Cu (s)) Mg (s)+Cu. SO 4 (aq)+2 e- Mg. SO 4 (aq)+Cu (s)+2 e-

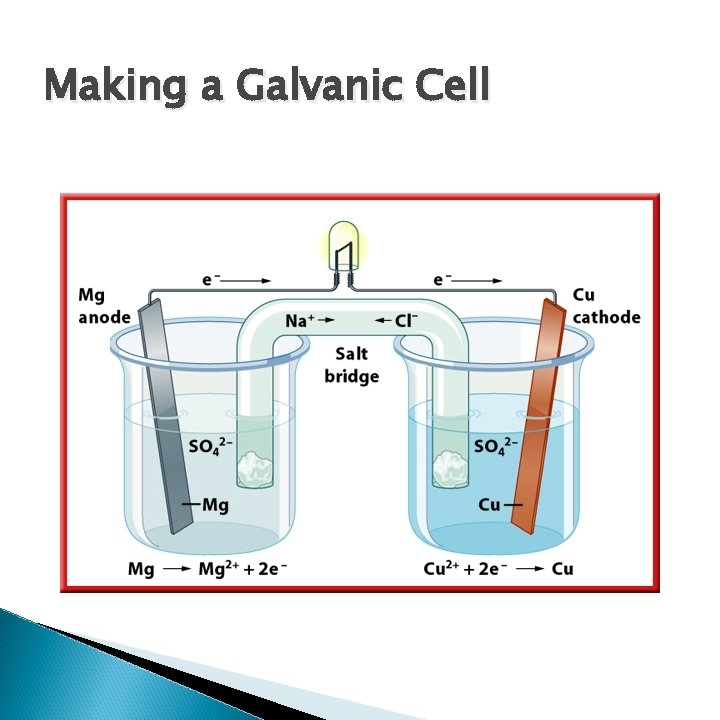
Making a Galvanic Cell

Making a Galvanic Cell Anode Cathode Salt Bridge Flow of electrons

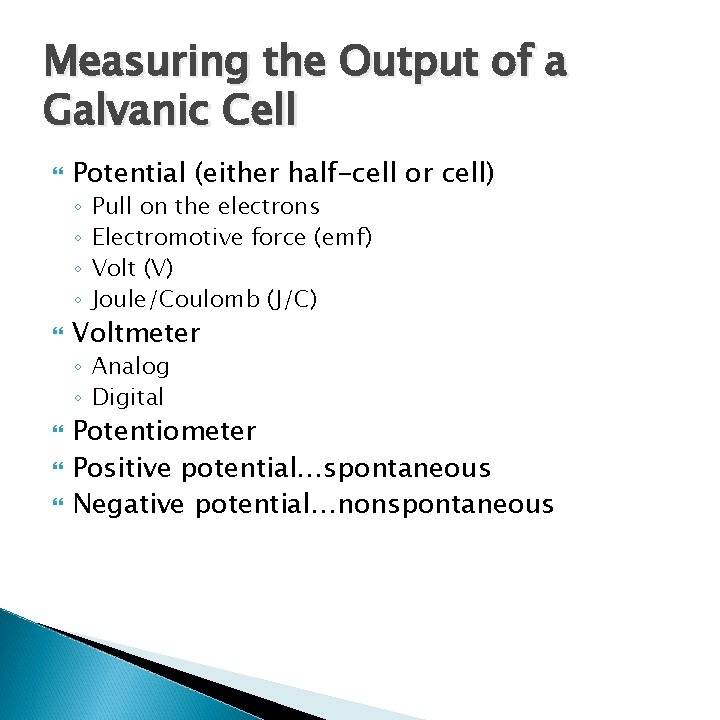
Measuring the Output of a Galvanic Cell Potential (either half-cell or cell) ◦ ◦ Pull on the electrons Electromotive force (emf) Volt (V) Joule/Coulomb (J/C) Voltmeter ◦ Analog ◦ Digital Potentiometer Positive potential…spontaneous Negative potential…nonspontaneous

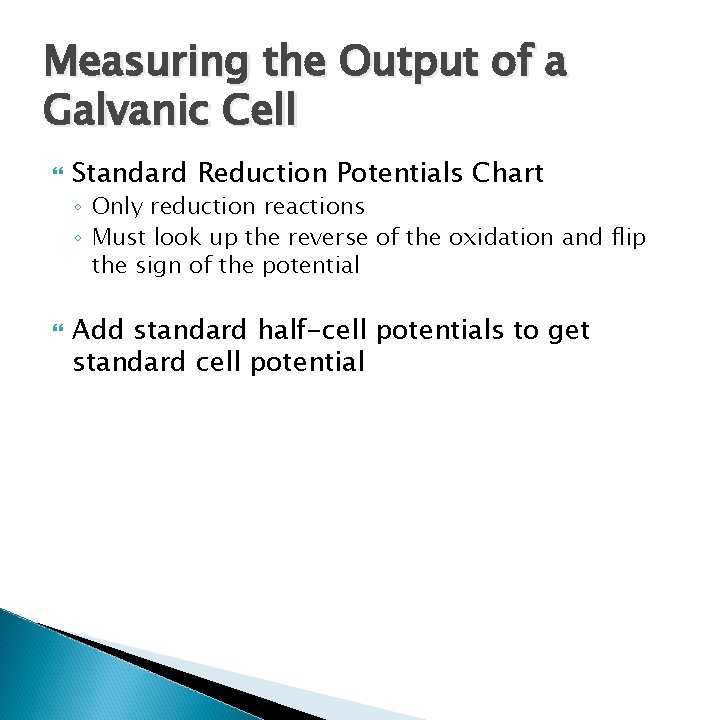
Measuring the Output of a Galvanic Cell Standard Reduction Potentials Chart ◦ Only reduction reactions ◦ Must look up the reverse of the oxidation and flip the sign of the potential Add standard half-cell potentials to get standard cell potential

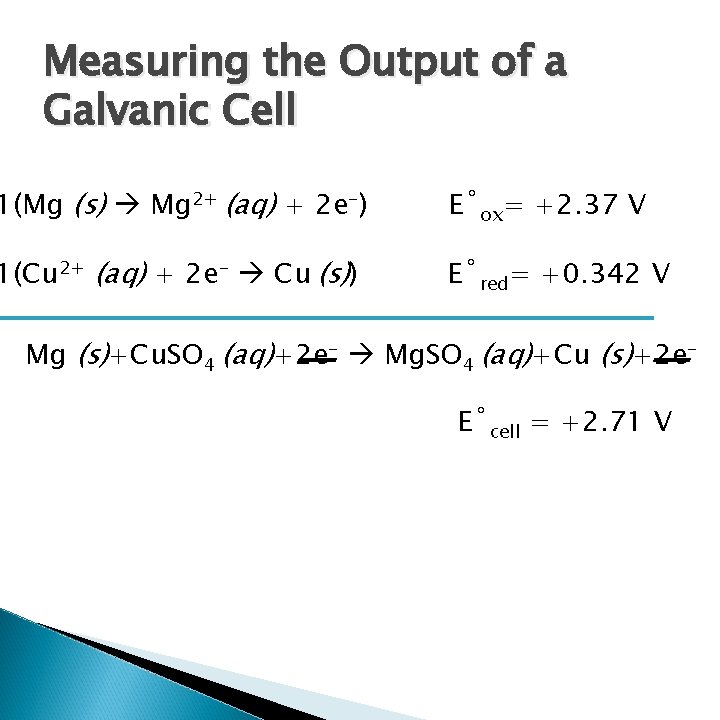
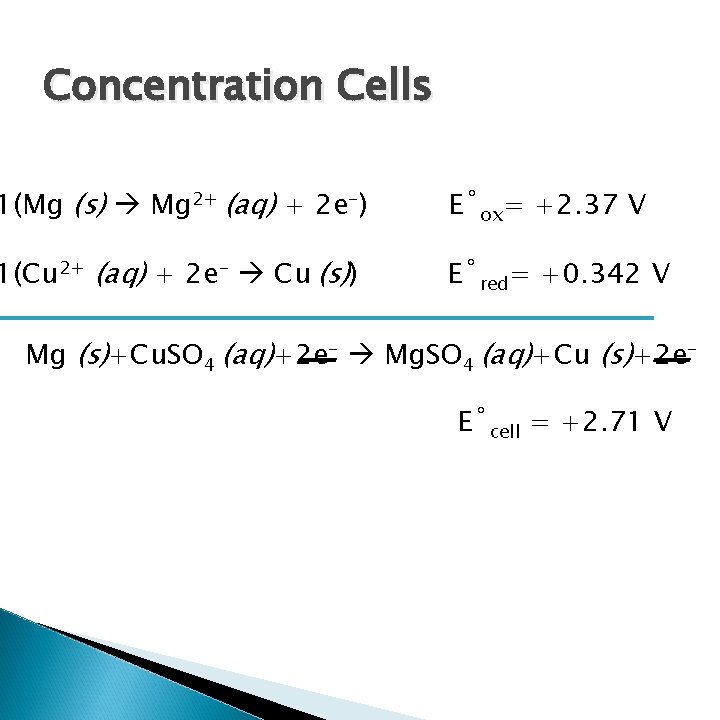
Measuring the Output of a Galvanic Cell 1(Mg (s) Mg 2+ (aq) + 2 e-) E˚ox= +2. 37 V 1(Cu 2+ (aq) + 2 e- Cu (s)) E˚red= +0. 342 V Mg (s)+Cu. SO 4 (aq)+2 e- Mg. SO 4 (aq)+Cu (s)+2 e. E˚cell = +2. 71 V

Writing a Line Notation Oxidation||Reduction X(s)|X+(aq)||Y+(aq)|Y(s) Mg(s)|Mg 2+(aq)||Cu 2+(aq)|Cu(s) 1(Mg (s) Mg 2+ (aq) + 2 e-) 1(Cu 2+ (aq) + 2 e- Cu (s)) Mg (s)+Cu. SO 4 (aq)+2 e- Mg. SO 4 (aq)+Cu (s)+2 e-

Writing a Line Notation Cu(s)|Cu 2+(aq)||Ag 1+(aq)|Ag(s) 1(Cu (s) Cu 2+ (aq) + 2 e-) E˚ox= -0. 342 V 2(Ag 1+ (aq) + 1 e- Ag (s)) E˚red= +0. 800 V Cu(s)+2 Ag 1+(aq)+2 e- Cu 2+(aq)+2 Ag (s)+2 e. E˚cell= +0. 458 V

Batteries Series of electrochemical cells connected to each other Completes the circuit Dry cell ◦ Flashlight battery ◦ Watch battery Wet Cell ◦ Car battery

Batteries Carbon-Zinc Battery ◦ ◦ Zinc casing…anode Carbon rod…cathode Mn. O 2 is actually reduced Alkaline battery…has KOH rather than NH 4 Cl

Batteries Carbon-Zinc Battery ◦ Zn(s) Zn 2+(aq) + 2 e- ◦ 2 NH 41+(aq) + 2 Mn. O 2(s) + 2 e- Mn 2 O 3(s) + 2 NH 3(g) + H 2 O(l)

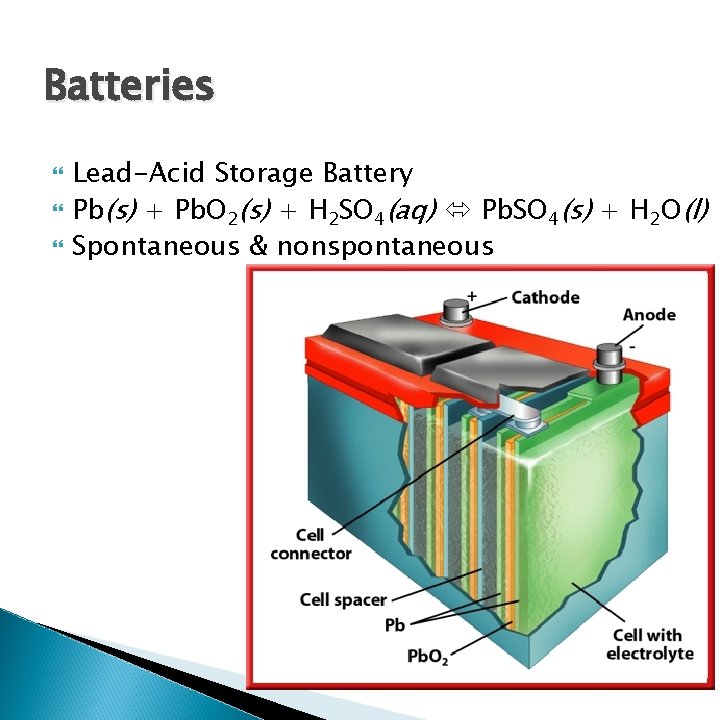
Batteries Lead-Acid Storage Battery Pb(s) + Pb. O 2(s) + H 2 SO 4(aq) Pb. SO 4(s) + H 2 O(l) Spontaneous & nonspontaneous

Concentration Cells Not 1 M Require additional calculations Can manipulate potential to a particular V

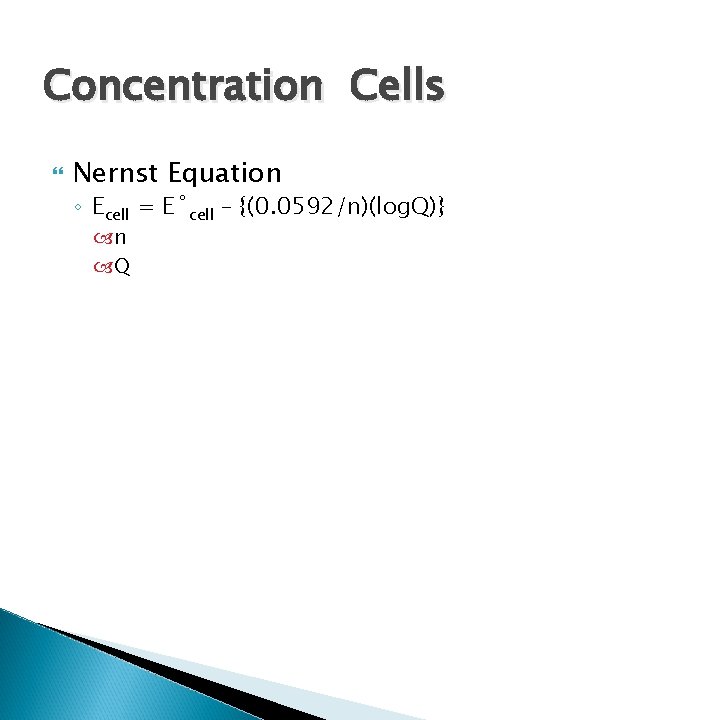
Concentration Cells Nernst Equation ◦ Ecell = E˚cell – {(0. 0592/n)(log. Q)} n Q

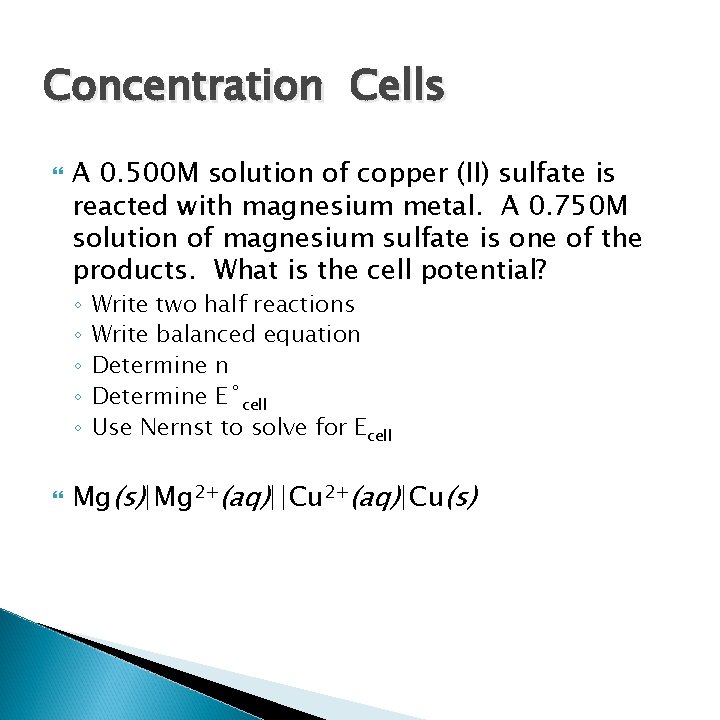
Concentration Cells A 0. 500 M solution of copper (II) sulfate is reacted with magnesium metal. A 0. 750 M solution of magnesium sulfate is one of the products. What is the cell potential? ◦ ◦ ◦ Write two half reactions Write balanced equation Determine E˚cell Use Nernst to solve for Ecell Mg(s)|Mg 2+(aq)||Cu 2+(aq)|Cu(s)

Concentration Cells 1(Mg (s) Mg 2+ (aq) + 2 e-) E˚ox= +2. 37 V 1(Cu 2+ (aq) + 2 e- Cu (s)) E˚red= +0. 342 V Mg (s)+Cu. SO 4 (aq)+2 e- Mg. SO 4 (aq)+Cu (s)+2 e. E˚cell = +2. 71 V
![Concentration Cells Ecell 2 71 V 0 05922log0 750 5 Ecell Concentration Cells Ecell = 2. 71 V – {(0. 0592/2)(log([0. 75]/[0. 5]))} Ecell =](https://slidetodoc.com/presentation_image_h2/25c1116fee6fe3b9ffb0f3ddabaec40a/image-25.jpg)
Concentration Cells Ecell = 2. 71 V – {(0. 0592/2)(log([0. 75]/[0. 5]))} Ecell = 2. 71 V – {(0. 0296)(0. 176)} Ecell = 2. 71 V – 0. 00521 Ecell = 2. 70 V

Concentration Cells Cu(s)|Cu 2+(0. 0100 M)||Ag 1+(0. 0250 M)|Ag(s)