Electrochemistry Part III Reduction Potentials Jespersen Chap 20















- Slides: 25

Electrochemistry Part III: Reduction Potentials Jespersen Chap. 20 Sec 2 & 3 Dr. C. Yau Fall 2014 1

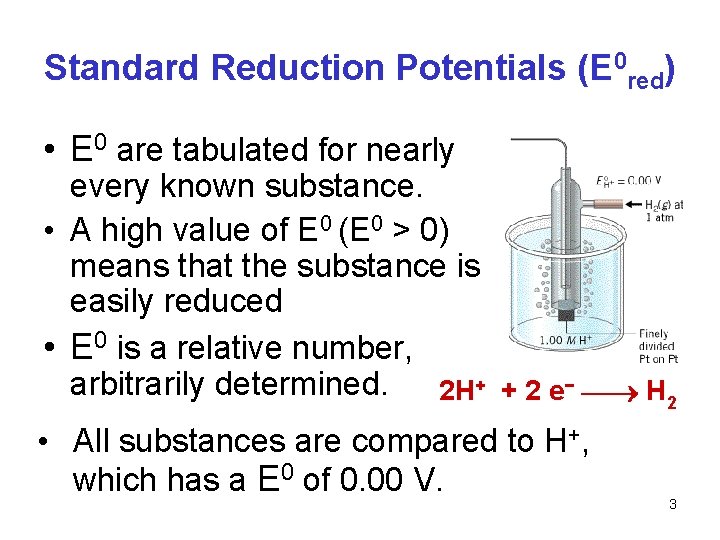
Electrical Potential • Every substance has the potential to gain electrons, or be reduced in oxidation state • The relative ease of gaining electrons is termed the reduction potential, and is symbolized Ered • If the matter being observed is in standard state then E is termed the standard reduction potential and is symbolized as E 0 red. • (Often "red" is omitted and given as Eo) • Units are V (volts) • 1 V=1 J/C where C is coulomb, a unit of charge. 2

Standard Reduction Potentials (E 0 red) • E 0 are tabulated for nearly every known substance. • A high value of E 0 (E 0 > 0) means that the substance is easily reduced • E 0 is a relative number, arbitrarily determined. 2 H+ + 2 e− H 2 • All substances are compared to H+, which has a E 0 of 0. 00 V. 3

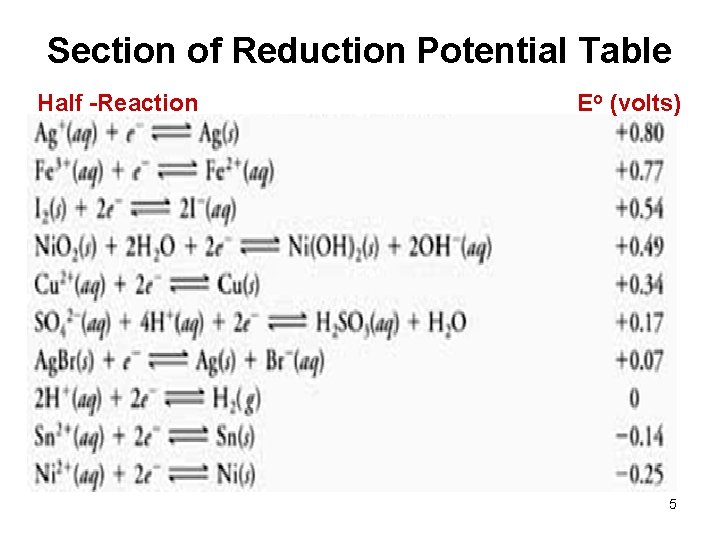
Table 20. 1 p. 928 4

Section of Reduction Potential Table Half -Reaction Eo (volts) 5

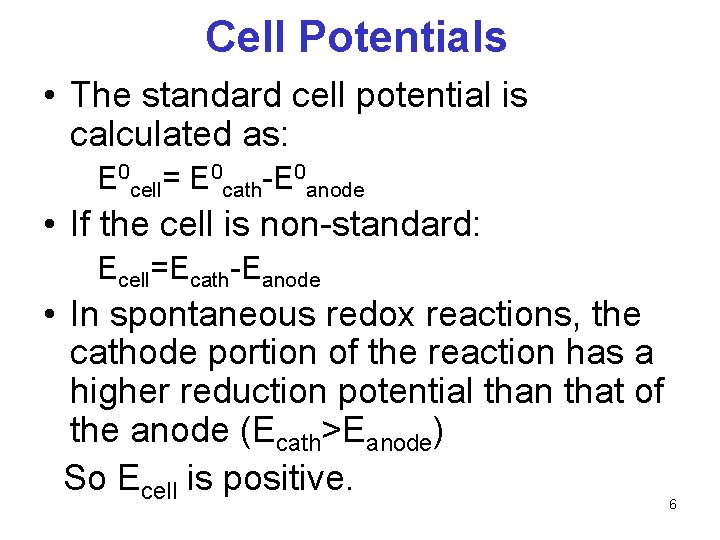
Cell Potentials • The standard cell potential is calculated as: E 0 cell= E 0 cath-E 0 anode • If the cell is non-standard: Ecell=Ecath-Eanode • In spontaneous redox reactions, the cathode portion of the reaction has a higher reduction potential than that of the anode (Ecath>Eanode) So Ecell is positive. 6

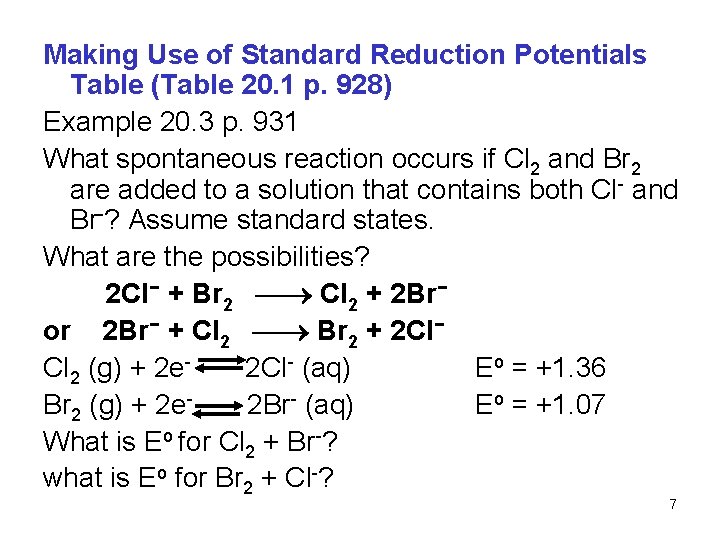
Making Use of Standard Reduction Potentials Table (Table 20. 1 p. 928) Example 20. 3 p. 931 What spontaneous reaction occurs if Cl 2 and Br 2 are added to a solution that contains both Cl- and Br-? Assume standard states. What are the possibilities? 2 Cl− + Br 2 Cl 2 + 2 Br− or 2 Br− + Cl 2 Br 2 + 2 Cl− Cl 2 (g) + 2 e 2 Cl- (aq) Eo = +1. 36 Br 2 (g) + 2 e 2 Br- (aq) Eo = +1. 07 What is Eo for Cl 2 + Br-? what is Eo for Br 2 + Cl-? 7

Pract Exer 20. 7 p. 932 Predict whether the following reaction is spontaneous if all the ions are 1. 0 M at 25 o. C. If it is not, write the equation that represents a spontaneous reaction. Ni 2+(aq) + 2 Fe 2+(aq) Ni(s) + 2 Fe 3+ (aq) Which reaction is at the cathode? Do Pract Exer 5, 6 p. 932 8

Helpful Tips For a reaction to be spontaneous, the half-rxn with the larger (more positive) standard reduction potential Eo takes place as REDUCTION… the other half-rxn will be reversed and occur as oxidation (change the sign of E o) and ADD the two Eo together. This is the same as saying "to subtract one Eo from the other". 9

Example 20. 4 p. 932 A typical cell of a lead storage battery of the type used to start automobiles is constructed using electrodes made of lead and lead(IV) oxide Pb. O 2 and with sulfuric acid as the electrolyte. The half-rxns & their standard reduction potentials in this system are shown below. What is the cell rxn and what is the standard potential of the cell? Pb. SO 4 (s) + H+(aq) + 2 e. Pb(s) + HSO 4 (aq) Eo= -0. 36 V Pb. O 2(s)+3 H+(aq)+HSO 4 (aq)+ 2 e Pb. SO 4 (s)+2 H 2 O (l) Eo = 1. 69 V 10

Example 20. 5 p. 933 A galvanic cell is constructed by placing 1. 0 M aluminum nitrate in one beaker with an Al electrode and a second beaker with 1. 0 M copper(II) nitrate and a Cu electrode. When a saltbridge is inserted to connect the 2 beakers, what cell potential do we expect to measure between the Cu and Al electrodes? Which electrode is the anode, and what is the spontaneous chemical reaction? Note: We do not multiply the Eo by two. Do Pract Exer 8, 9, 10 p. 934 11

Example 20. 6 p. 935 Determine whether the following rxns, at standard state, are spontaneous as written. If not, write the eqn for the rxn that is. 1) Cu (s) + 2 H+ (aq) Cu 2+ (aq) + H 2 (g) 2) 3 Cu(s)+ 2 NO 3 (aq)+ 8 H+(aq) 3 Cu 2+(aq)+ 2 NO(g) + 4 H 2 O(l) Do Pract Exer 11, 12 p. 936 12

Redox Trends in Periodic Table Be sure to review Sec 9. 7 p. 381 Reactivities of Elements & Electronegativity Reactivities of metals and nonmetals are related to electronegativity. Metals react by losing electrons to form cations. Is this oxidation or reduction? Write a general eqn for metals reacting. The most “reactive metal” or “active metal” are ones that can lose electrons most readily (the most electronegative or least? ) What is the general trend of ease of oxidation? General trend of ease of reduction? 13

Redox Trends in Periodic Table Nonmetals react by gaining electrons to form anions. Is this oxidation or reduction? Write a general equation of a nonmetals reacting, using Group VIIA as an example. The most reactive nonmetals are ones that can gain electrons most readily (the most electronegative or least? ) What is the general trend of ease of reduction? General trend of ease of oxidation? 14

What do metals tend to do? Metals have tendencies to. . . M M+ a) be oxidized. b) be reduced. Metals tend to be a) oxidizing agents. b) reducing agents. 15

What do nonmetals tend to do? Nonmetals with high electronegativities have strong tendencies to. . . a)be oxidized. X Xb)be reduced. Which two elements are the most powerful oxidizing agent? Ans. F 2 followed by O 2 16

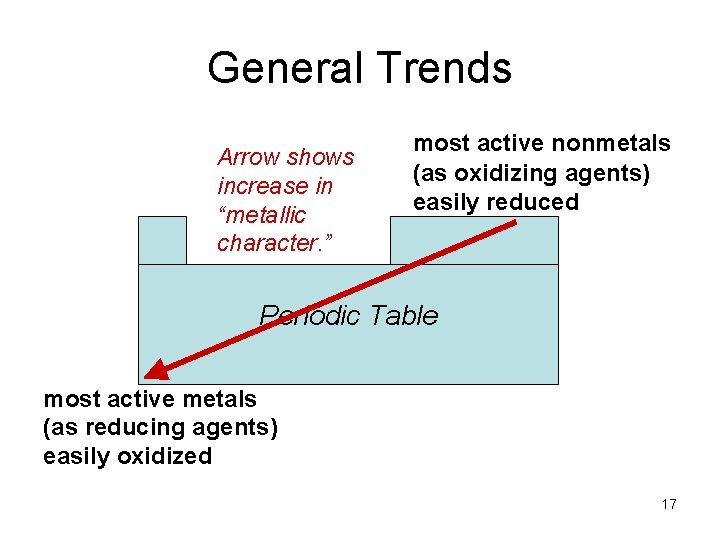
General Trends Arrow shows increase in “metallic character. ” most active nonmetals (as oxidizing agents) easily reduced Periodic Table most active metals (as reducing agents) easily oxidized 17

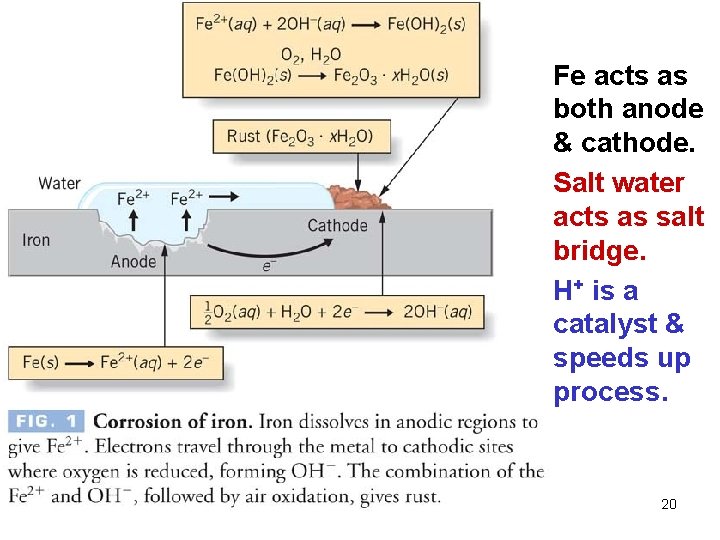
Protection Against Corrosion What exactly is "corrosion" of a metal? We often talk about a metal being "oxidized. " When an iron nail rusts, what exactly is it doing? What is rust? A simplified view of the rusting process: Fe (s) Fe 2 O 3 (s) oxidation or reduction? Why does iron rusts more quickly in contact with a metal such as Cu? It rusts more slowly in contact with other metals…such as what? 18

Facts about Rusting of Iron • does not rust in dry air (moisture must be present) • does not rust in air-free water (O 2 must be present) • loss of Fe and deposit of rust often occur at different places on the same object • rusts more quickly at low p. H • rusts more quickly in contact with ionic solutions • rusts more quickly in contact with a less active metal such as Cu • rusts more slowly in contact with a more active 19 metal such as Zn or Mg.

Fe acts as both anode & cathode. Salt water acts as salt bridge. H+ is a catalyst & speeds up process. 20

Elimination of Corrosive Factors • Keep dry • Rinse off road salts (remove electrolytes) • Protect with paint (keep oxygen, moisture, salts and acids out) • What about contact with other metals? 21

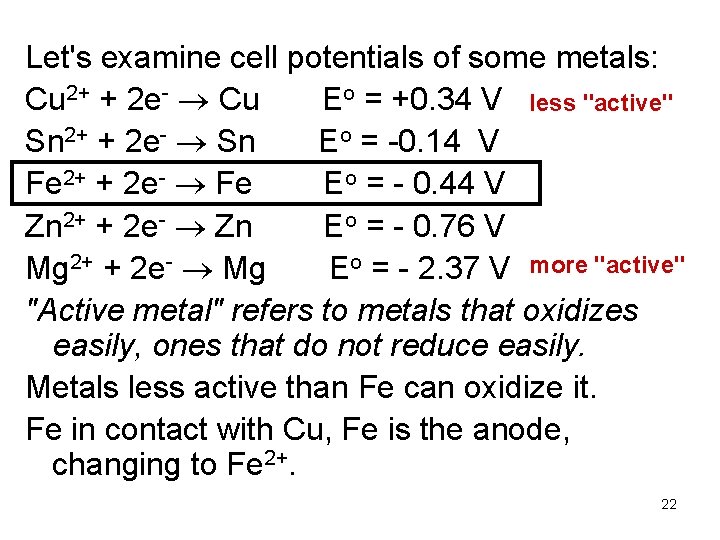
Let's examine cell potentials of some metals: Cu 2+ + 2 e- Cu Eo = +0. 34 V less "active" Sn 2+ + 2 e- Sn Eo = -0. 14 V Fe 2+ + 2 e- Fe Eo = - 0. 44 V Zn 2+ + 2 e- Zn Eo = - 0. 76 V Mg 2+ + 2 e- Mg Eo = - 2. 37 V more "active" "Active metal" refers to metals that oxidizes easily, ones that do not reduce easily. Metals less active than Fe can oxidize it. Fe in contact with Cu, Fe is the anode, changing to Fe 2+. 22

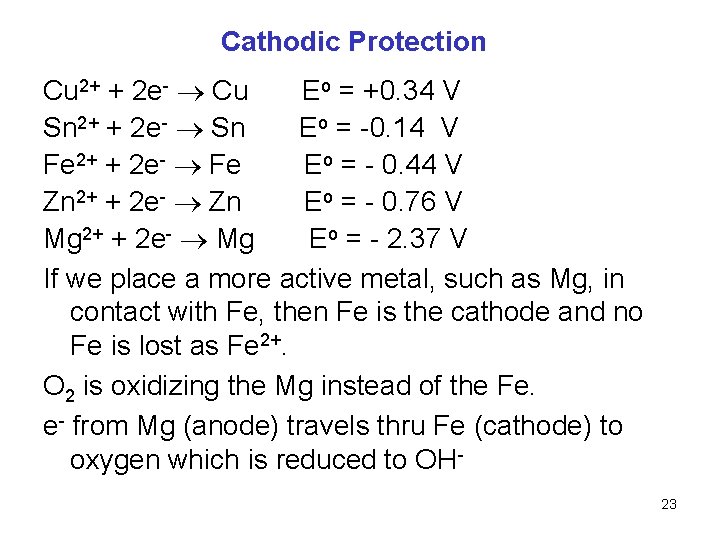
Cathodic Protection Cu 2+ + 2 e- Cu Eo = +0. 34 V Sn 2+ + 2 e- Sn Eo = -0. 14 V Fe 2+ + 2 e- Fe Eo = - 0. 44 V Zn 2+ + 2 e- Zn Eo = - 0. 76 V Mg 2+ + 2 e- Mg Eo = - 2. 37 V If we place a more active metal, such as Mg, in contact with Fe, then Fe is the cathode and no Fe is lost as Fe 2+. O 2 is oxidizing the Mg instead of the Fe. e- from Mg (anode) travels thru Fe (cathode) to oxygen which is reduced to OH 23

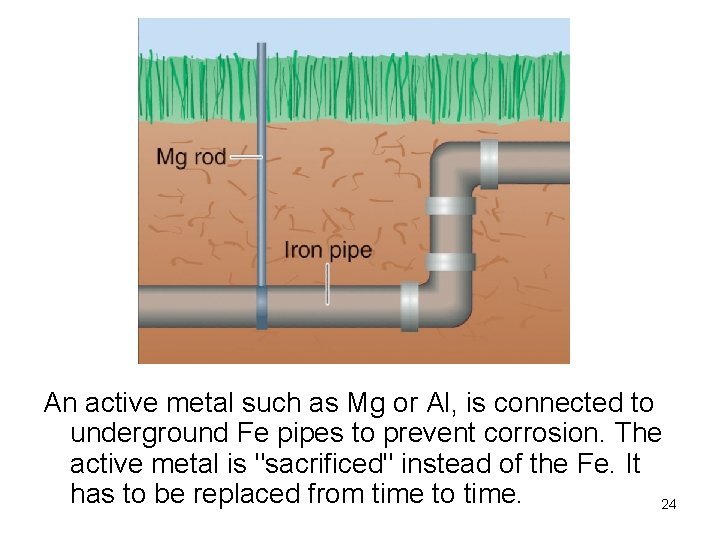
An active metal such as Mg or Al, is connected to underground Fe pipes to prevent corrosion. The active metal is "sacrificed" instead of the Fe. It has to be replaced from time to time. 24

What about other metals? How do you protect other metals? Cu 2+ + 2 e- Cu Eo = +0. 34 V Sn 2+ + 2 e- Sn Eo = -0. 14 V Fe 2+ + 2 e- Fe Eo = - 0. 44 V Zn 2+ + 2 e- Zn Eo = - 0. 76 V Mg 2+ + 2 e- Mg Eo = - 2. 37 V Just remember you would use a more active metal. What does "active" mean? “Sacrificial” metal is the more active metal. 25