Electrochemistry Lesson 7 The Standard Hydrogen Cell The

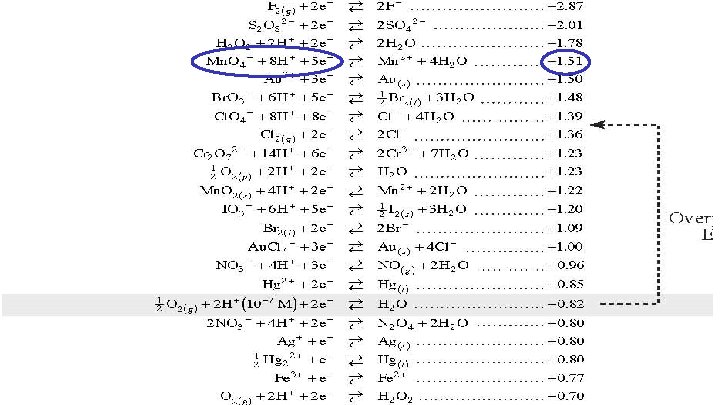
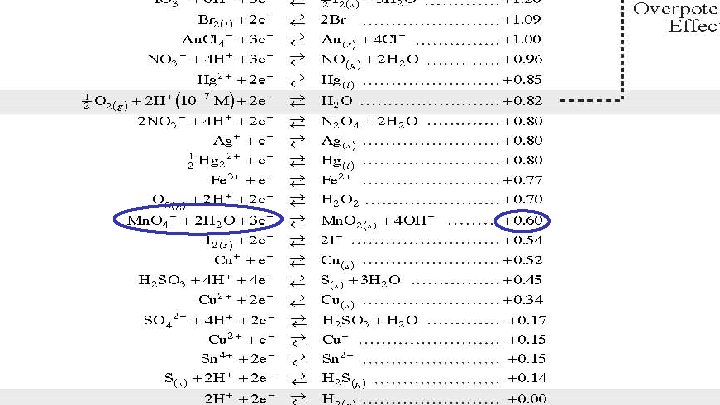


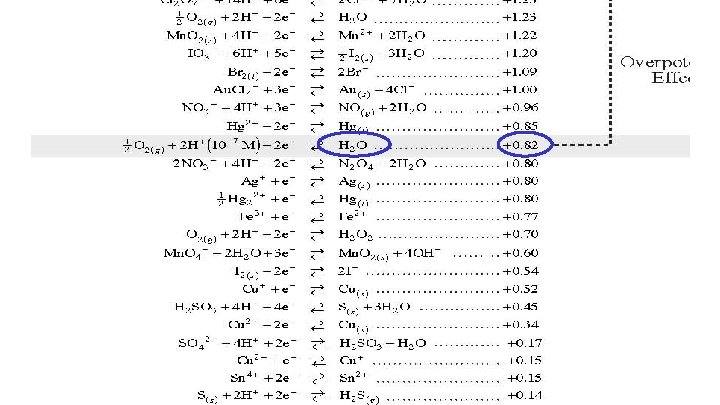
- Slides: 28

Electrochemistry Lesson 7 The Standard Hydrogen Cell

The Standard Hydrogen Half Cell The zero point of the reduction chart is the hydrogen half-cell. E is the cell potential or voltage E 0 is the standard cell potential @ 25 o. C solutions are 1. 0 M gases are 101 KPa

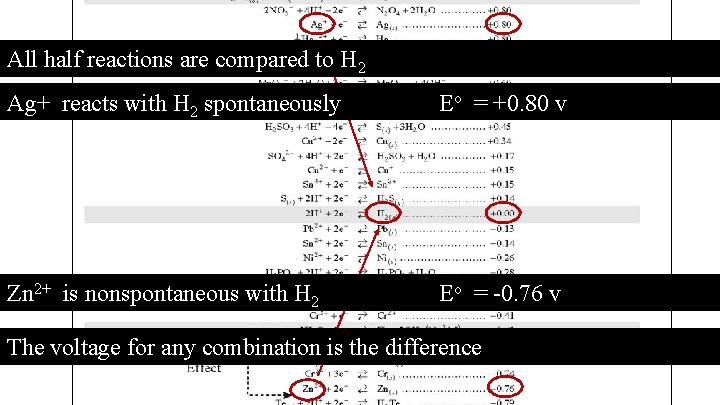
All half reactions are compared to H 2 Ag+ reacts with H 2 spontaneously Eo = +0. 80 v Zn 2+ is nonspontaneous with H 2 Eo = -0. 76 v The voltage for any combination is the difference

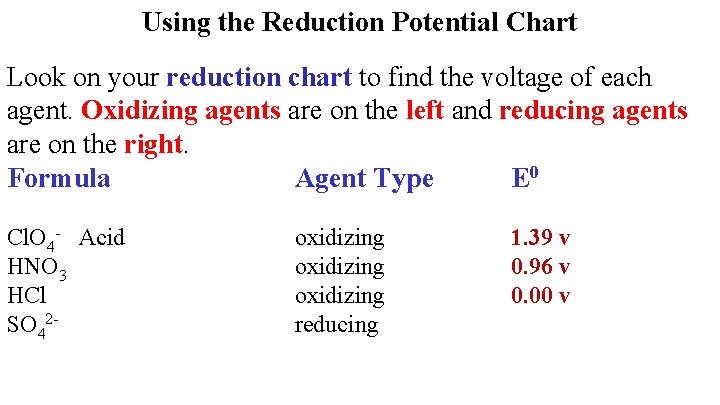
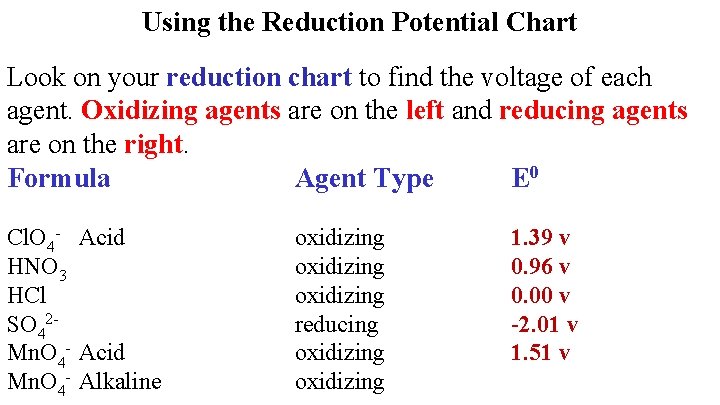
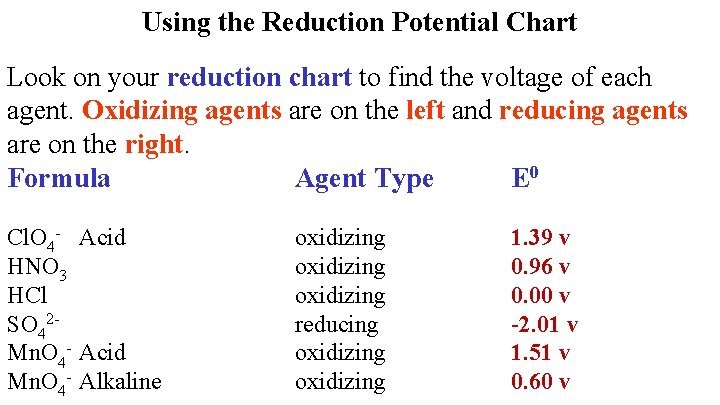
Using the Reduction Potential Chart Look on your reduction chart to find the voltage of each agent. Oxidizing agents are on the left and reducing agents are on the right. Formula Agent Type E 0 HCl. O 4 oxidizing


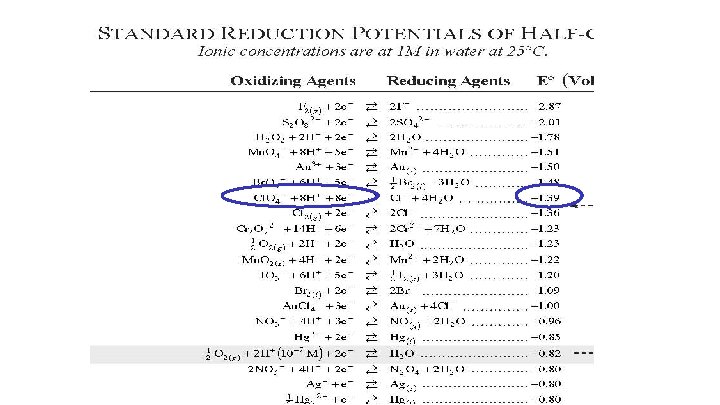
Using the Reduction Potential Chart Look on your reduction chart to find the voltage of each agent. Oxidizing agents are on the left and reducing agents are on the right. Formula Agent Type E 0 Cl. O 4 - Acid HNO 3 oxidizing 1. 39 v

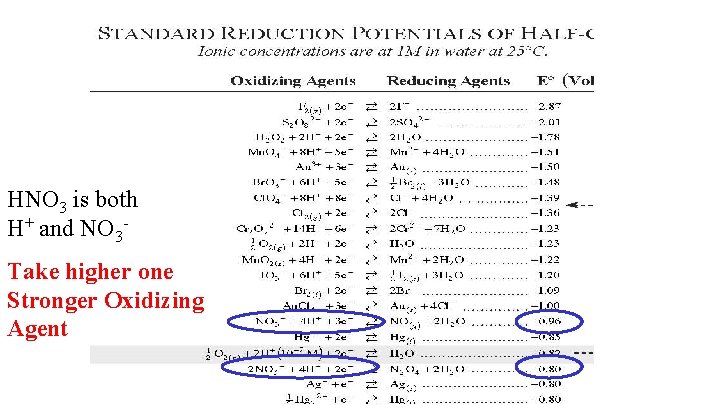
HNO 3 is both H+ and NO 3 Take higher one Stronger Oxidizing Agent

Using the Reduction Potential Chart Look on your reduction chart to find the voltage of each agent. Oxidizing agents are on the left and reducing agents are on the right. Formula Agent Type E 0 Cl. O 4 - Acid HNO 3 HCl oxidizing 1. 39 v 0. 96 v


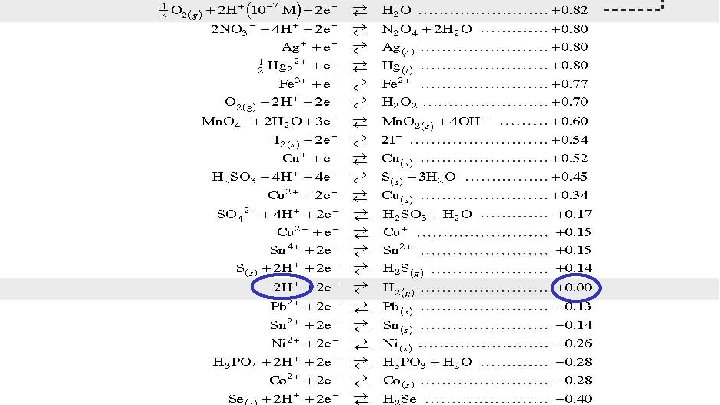
Using the Reduction Potential Chart Look on your reduction chart to find the voltage of each agent. Oxidizing agents are on the left and reducing agents are on the right. Formula Agent Type E 0 Cl. O 4 - Acid HNO 3 HCl SO 42 - oxidizing reducing 1. 39 v 0. 96 v 0. 00 v

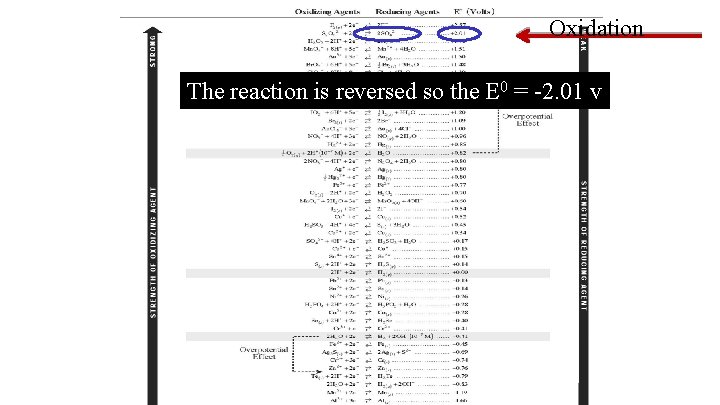
Oxidation The reaction is reversed so the E 0 = -2. 01 v

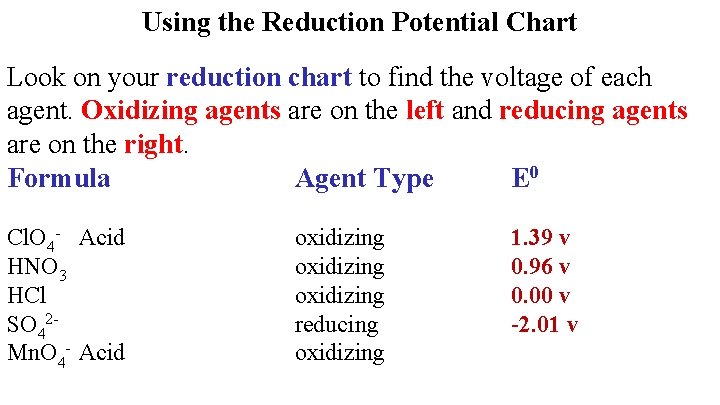
Using the Reduction Potential Chart Look on your reduction chart to find the voltage of each agent. Oxidizing agents are on the left and reducing agents are on the right. Formula Agent Type E 0 Cl. O 4 - Acid HNO 3 HCl SO 42 Mn. O 4 - Acid oxidizing reducing oxidizing 1. 39 v 0. 96 v 0. 00 v -2. 01 v
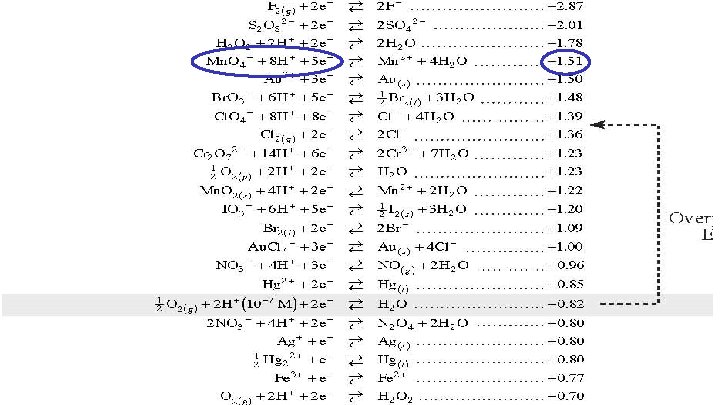

Using the Reduction Potential Chart Look on your reduction chart to find the voltage of each agent. Oxidizing agents are on the left and reducing agents are on the right. Formula Agent Type E 0 Cl. O 4 - Acid HNO 3 HCl SO 42 Mn. O 4 - Acid Mn. O 4 - Alkaline oxidizing reducing oxidizing 1. 39 v 0. 96 v 0. 00 v -2. 01 v 1. 51 v
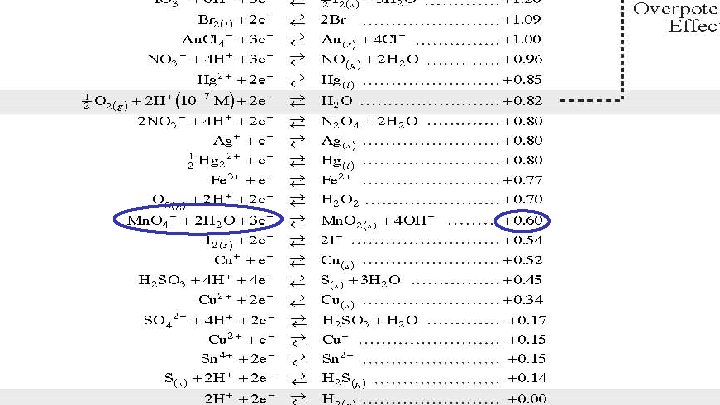

Using the Reduction Potential Chart Look on your reduction chart to find the voltage of each agent. Oxidizing agents are on the left and reducing agents are on the right. Formula Agent Type E 0 Cl. O 4 - Acid HNO 3 HCl SO 42 Mn. O 4 - Acid Mn. O 4 - Alkaline oxidizing reducing oxidizing 1. 39 v 0. 96 v 0. 00 v -2. 01 v 1. 51 v 0. 60 v

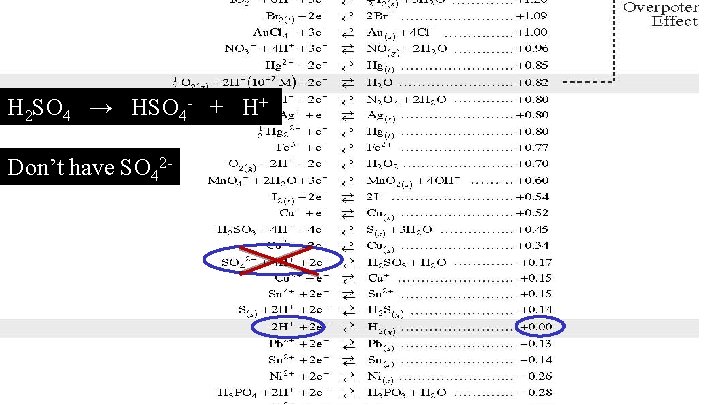
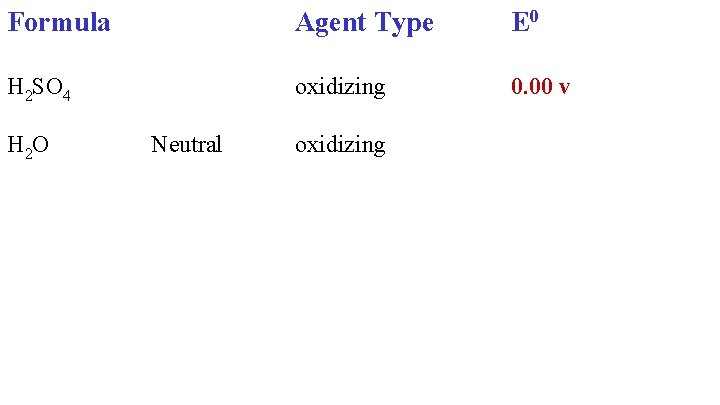
Formula Agent Type H 2 SO 4 oxidizing E 0

H 2 SO 4 → HSO 4 - + H+ Don’t have SO 42 -

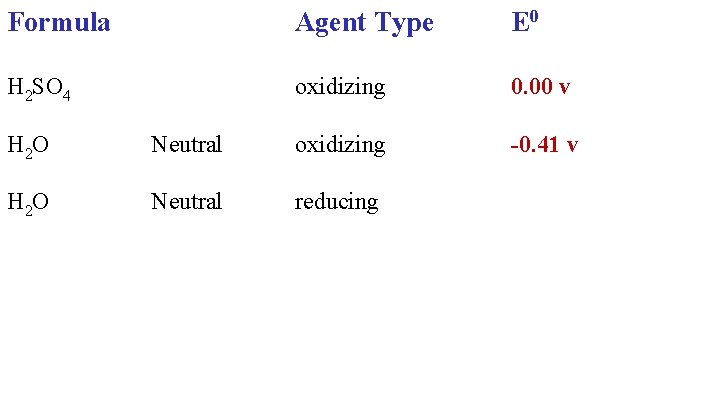
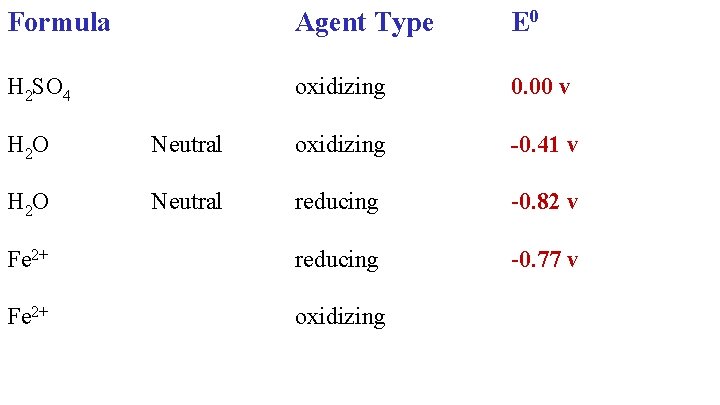
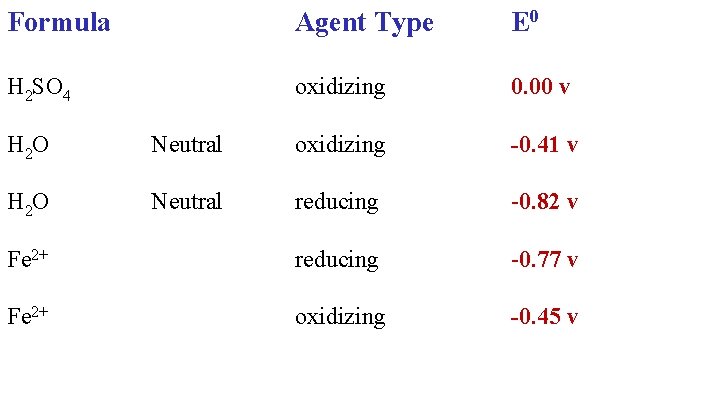
Formula Agent Type E 0 H 2 SO 4 oxidizing 0. 00 v H 2 O Neutral oxidizing


Formula Agent Type E 0 H 2 SO 4 oxidizing 0. 00 v -0. 41 v H 2 O Neutral oxidizing H 2 O Neutral reducing
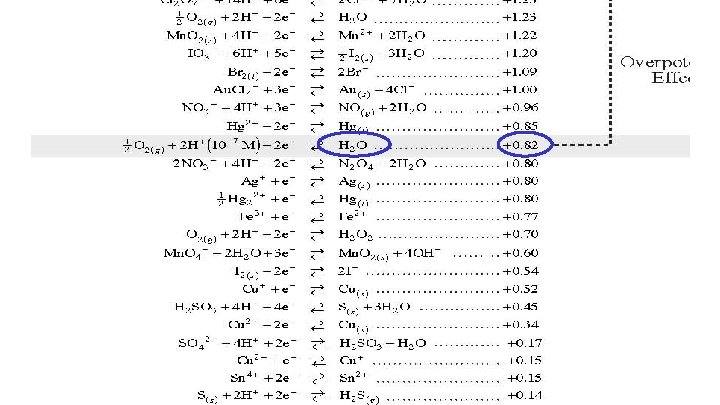

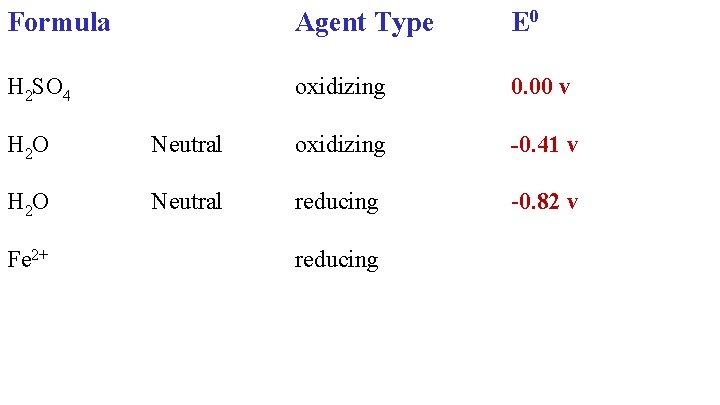
Formula Agent Type E 0 H 2 SO 4 oxidizing 0. 00 v H 2 O Neutral oxidizing -0. 41 v H 2 O Neutral reducing -0. 82 v Fe 2+ reducing

Formula Agent Type E 0 H 2 SO 4 oxidizing 0. 00 v H 2 O Neutral oxidizing -0. 41 v H 2 O Neutral reducing -0. 82 v Fe 2+ reducing -0. 77 v Fe 2+ oxidizing

Formula Agent Type E 0 H 2 SO 4 oxidizing 0. 00 v H 2 O Neutral oxidizing -0. 41 v H 2 O Neutral reducing -0. 82 v Fe 2+ reducing -0. 77 v Fe 2+ oxidizing -0. 45 v

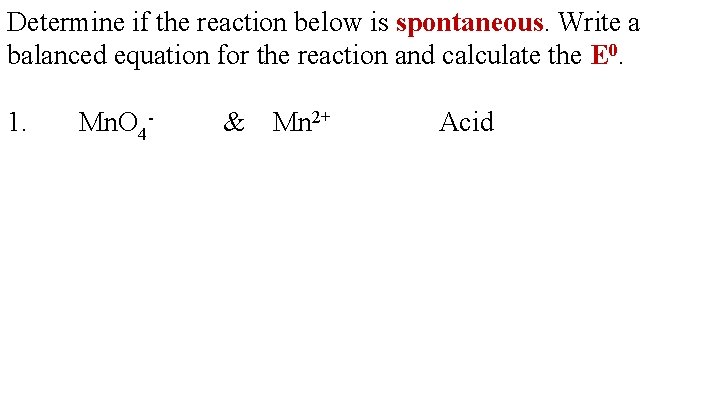
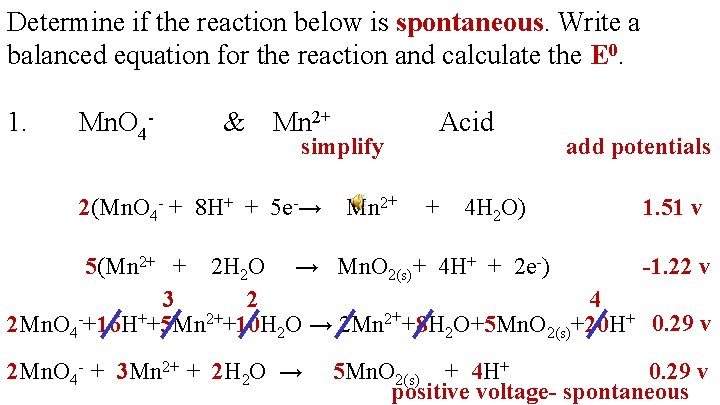
Determine if the reaction below is spontaneous. Write a balanced equation for the reaction and calculate the E 0. 1. Mn. O 4 - & Mn 2+ Acid

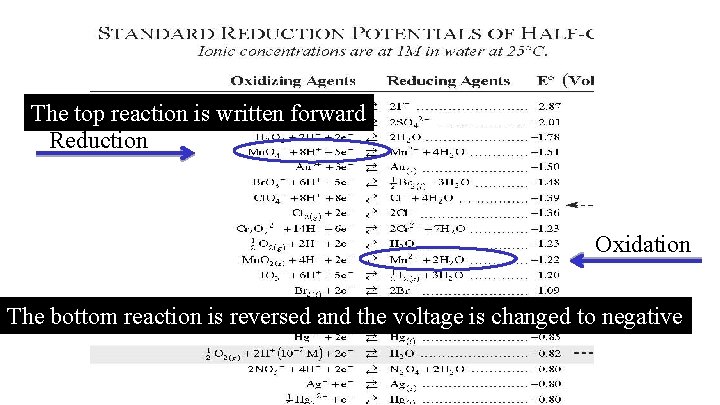
The top reaction is written forward Reduction Oxidation The bottom reaction is reversed and the voltage is changed to negative

Determine if the reaction below is spontaneous. Write a balanced equation for the reaction and calculate the E 0. 1. Mn. O 4 - & Mn 2+ simplify 2(Mn. O 4 - + 8 H+ + 5 e-→ Mn 2+ Acid + 4 H 2 O) add potentials 1. 51 v 5(Mn 2+ + 2 H 2 O → Mn. O 2(s)+ 4 H+ + 2 e-) -1. 22 v 3 2 4 2 Mn. O 4 -+16 H++5 Mn 2++10 H 2 O → 2 Mn 2++8 H 2 O+5 Mn. O 2(s)+20 H+ 0. 29 v 2 Mn. O 4 - + 3 Mn 2+ + 2 H 2 O → 5 Mn. O 2(s) + 4 H+ 0. 29 v positive voltage- spontaneous