Electrochemistry Lesson 2 Strength of Oxidizing Agent Lab

Electrochemistry Lesson 2 Strength of Oxidizing Agent Lab

Oxidation Agents Lab Notes Spontaneous Reactions: Sn(s) +Au(NO 3)3(aq)

Oxidation Agents Lab Notes Spontaneous Reactions: Sn(s) +Au(NO 3)3(aq) Sn(s) Sn 2+ + 2 e-
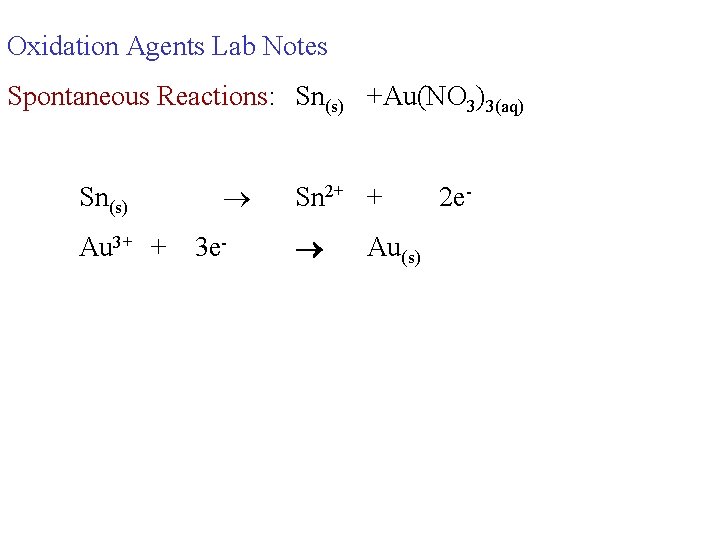
Oxidation Agents Lab Notes Spontaneous Reactions: Sn(s) +Au(NO 3)3(aq) Sn(s) Au 3+ + 3 e- Sn 2+ + Au(s) 2 e-
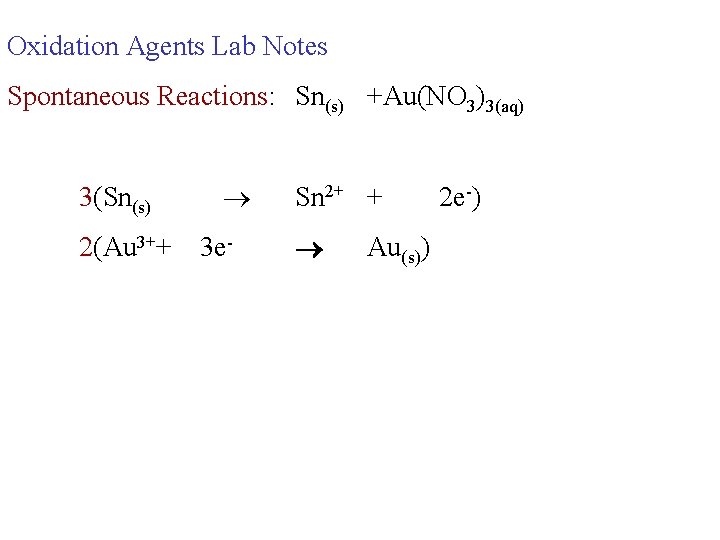
Oxidation Agents Lab Notes Spontaneous Reactions: Sn(s) +Au(NO 3)3(aq) 3(Sn(s) 2(Au 3++ 3 e- Sn 2+ + Au(s)) 2 e-)

Oxidation Agents Lab Notes Spontaneous Reactions: Sn(s) +Au(NO 3)3(aq) 3(Sn(s) 2(Au 3++ 3 Sn(s) + 3 e 2 Au 3+ Sn 2+ + 2 e-) Au(s)) 3 Sn 2+ + 2 Au(s)
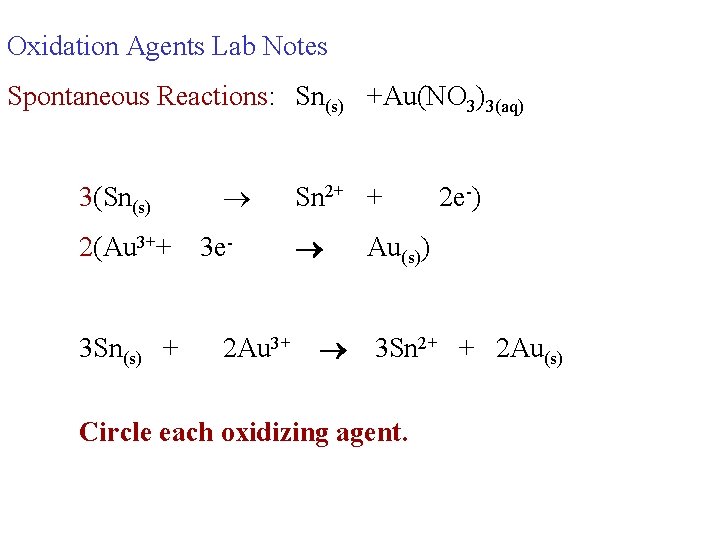
Oxidation Agents Lab Notes Spontaneous Reactions: Sn(s) +Au(NO 3)3(aq) 3(Sn(s) 2(Au 3++ 3 Sn(s) + 3 e 2 Au 3+ Sn 2+ + 2 e-) Au(s)) 3 Sn 2+ + 2 Au(s) Circle each oxidizing agent.

Oxidation Agents Lab Notes Spontaneous Reactions: Sn(s) +Au(NO 3)3(aq) 3(Sn(s) 2(Au 3++ 3 Sn(s) + 3 e 2 Au 3+ Sn 2+ + 2 e-) Au(s)) 3 Sn 2+ + 2 Au(s) Circle each oxidizing agent.
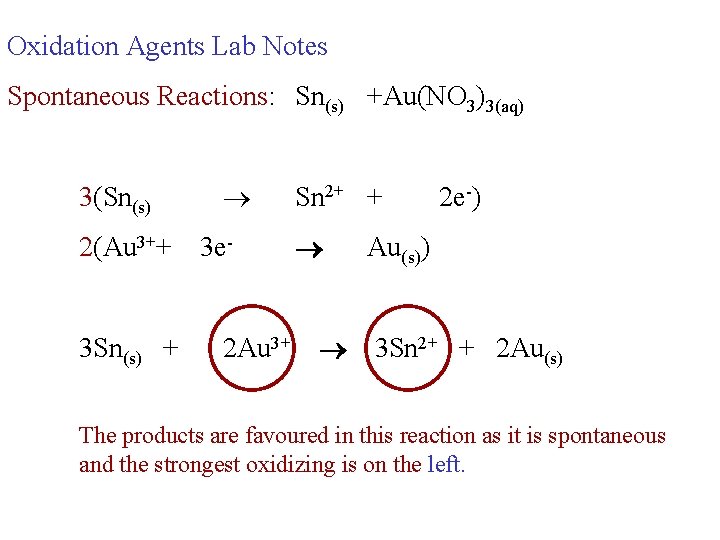
Oxidation Agents Lab Notes Spontaneous Reactions: Sn(s) +Au(NO 3)3(aq) 3(Sn(s) 2(Au 3++ 3 Sn(s) + 3 e 2 Au 3+ Sn 2+ + 2 e-) Au(s)) 3 Sn 2+ + 2 Au(s) The products are favoured in this reaction as it is spontaneous and the strongest oxidizing is on the left.
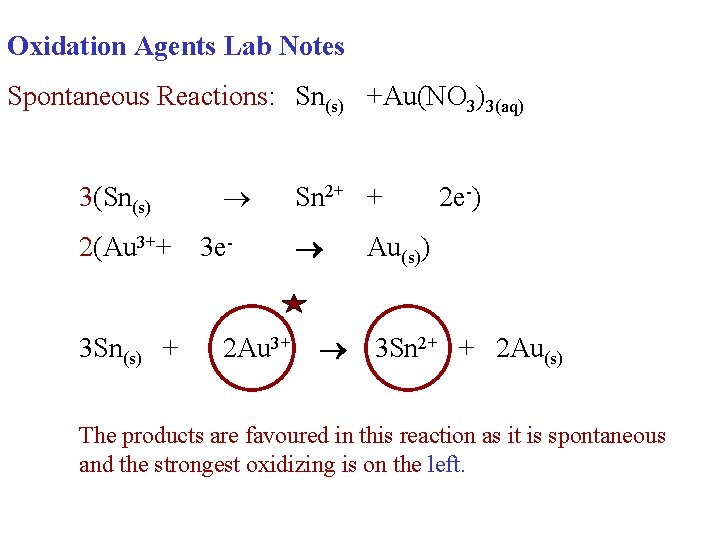
Oxidation Agents Lab Notes Spontaneous Reactions: Sn(s) +Au(NO 3)3(aq) 3(Sn(s) 2(Au 3++ 3 Sn(s) + 3 e 2 Au 3+ Sn 2+ + 2 e-) Au(s)) 3 Sn 2+ + 2 Au(s) The products are favoured in this reaction as it is spontaneous and the strongest oxidizing is on the left.

Oxidation Agents Lab Notes Nonspontaneous Reactions: Sn(s) + Zn(NO 3)2(aq)

Oxidation Agents Lab Notes Nonspontaneous Reactions: Sn(s) + Zn 2+ Sn(s) + Zn(NO 3)2(aq)

Oxidation Agents Lab Notes Nonspontaneous Reactions: Sn(s) + Zn 2+ Sn(s) + Zn(NO 3)2(aq)
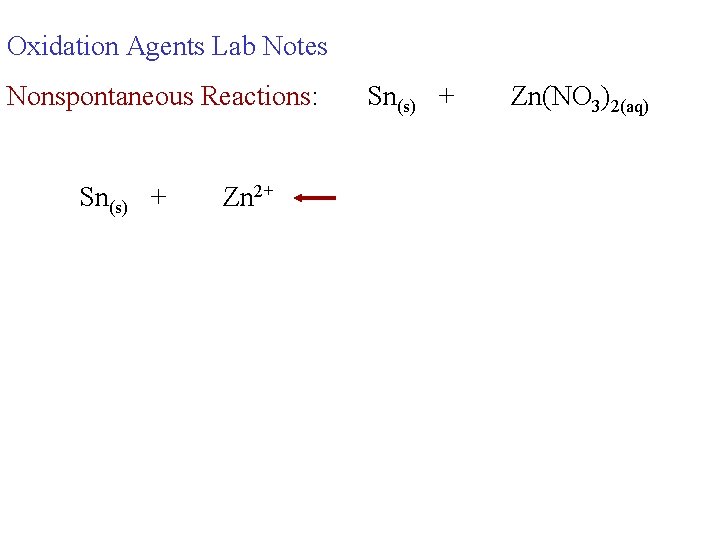
Oxidation Agents Lab Notes Nonspontaneous Reactions: Sn(s) + Zn 2+ Sn(s) + Zn(NO 3)2(aq)
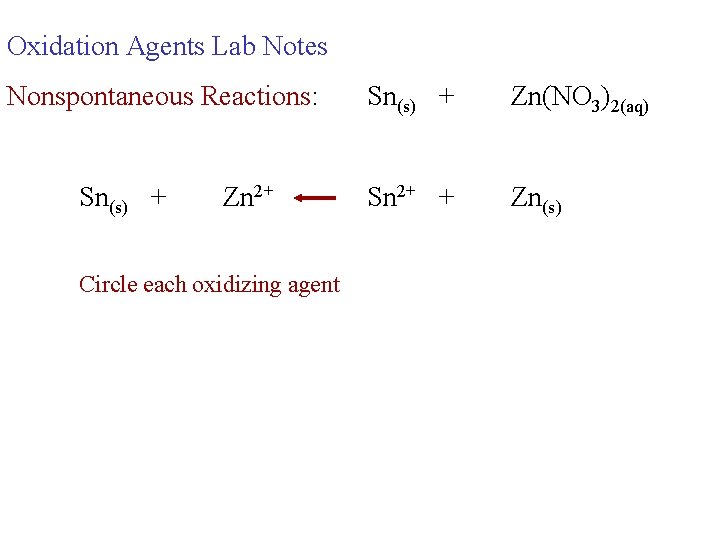
Oxidation Agents Lab Notes Nonspontaneous Reactions: Sn(s) + Zn 2+ Circle each oxidizing agent Sn(s) + Zn(NO 3)2(aq) Sn 2+ + Zn(s)
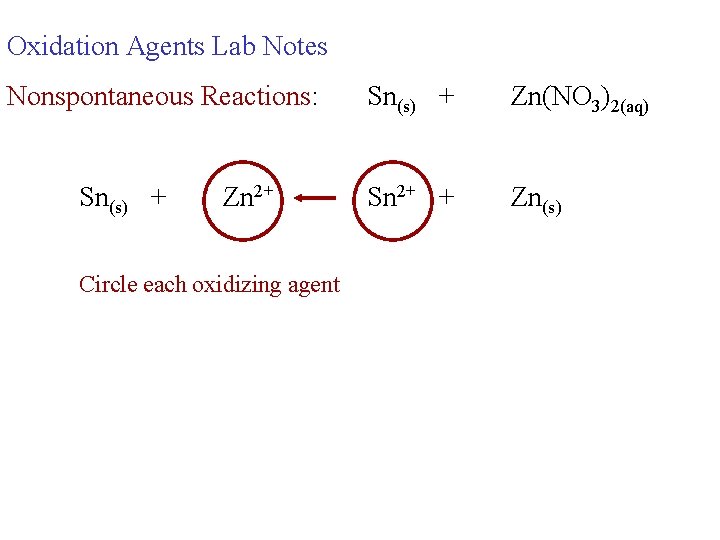
Oxidation Agents Lab Notes Nonspontaneous Reactions: Sn(s) + Zn 2+ Circle each oxidizing agent Sn(s) + Zn(NO 3)2(aq) Sn 2+ + Zn(s)

Oxidation Agents Lab Notes Nonspontaneous Reactions: Sn(s) + Zn 2+ Sn(s) + Zn(NO 3)2(aq) Sn 2+ + Zn(s) Circle each oxidizing agent the strongest oxidizing agent is on the right for a non-spontaneous reaction

Rank the oxiizing agents from strongest to weakest Au 3+ Sn 2+ Zn 2+
- Slides: 18