Electrochemistry Electrolytic Cells and Electrolysis o Electrolytic Cell





- Slides: 24

Electrochemistry

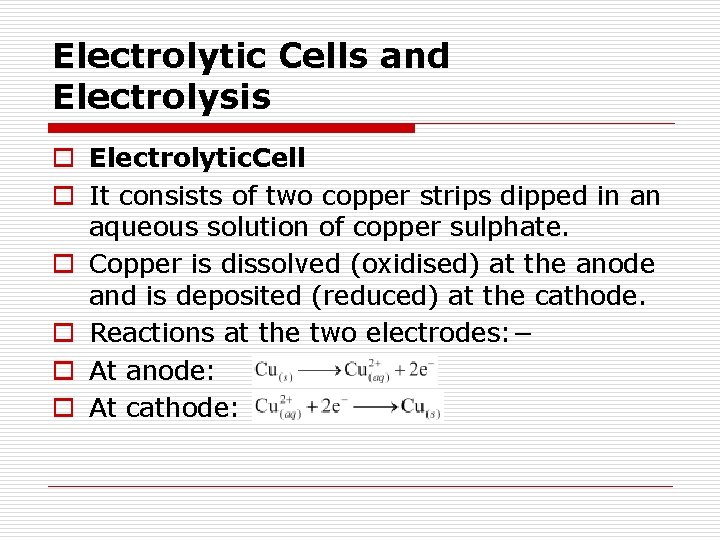
Electrolytic Cells and Electrolysis o Electrolytic. Cell o It consists of two copper strips dipped in an aqueous solution of copper sulphate. o Copper is dissolved (oxidised) at the anode and is deposited (reduced) at the cathode. o Reactions at the two electrodes: − o At anode: o At cathode:

Quantitative Aspects of Electrolysis o Faraday’s law of electrolysis o First law − The amount of chemical reaction which occurs at any electrode during electrolysis by a current is proportional to the quantity of electricity passed through the electrolytic solution or melt. o Second law − The amounts of different substances liberated by the same quantity of electricity passing through the electrolytic solution are proportional to their chemical equivalent weights.

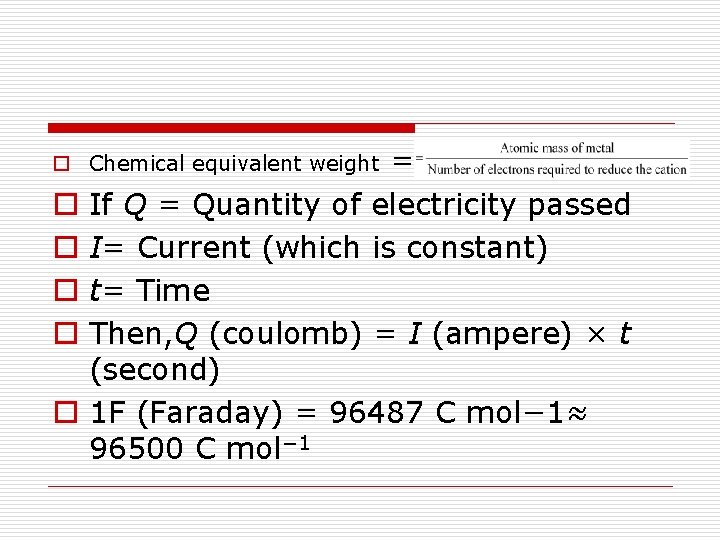
= If Q = Quantity of electricity passed I= Current (which is constant) t= Time Then, Q (coulomb) = I (ampere) × t (second) 1 F (Faraday) = 96487 C mol− 1≈ 96500 C mol− 1 o Chemical equivalent weight o o o

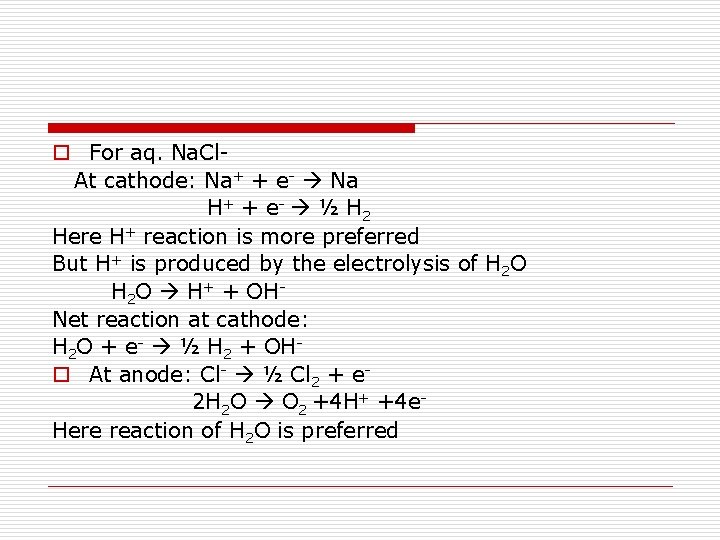
Products of Electrolysis o Products of electrolysis depend upon − o Nature of material being electrolysed o Type of electrodes: If inert (like Pt or Au), they act only as source or sink for electrons instead of participating in the reaction. On the other hand, if reactive, they participate in the reaction. Therefore, for different electrodes, the products of electrolysis may be different. o For example, the products of electrolysis of molten Na. Cl are Na metal and Cl 2 gas, while the products of electrolysis of aqueous solution of Na. Cl are Na. OH, Cl 2 and H 2.

o For aq. Na. Cl. At cathode: Na+ + e- Na H + + e - ½ H 2 Here H+ reaction is more preferred But H+ is produced by the electrolysis of H 2 O H+ + OHNet reaction at cathode: H 2 O + e- ½ H 2 + OHo At anode: Cl- ½ Cl 2 + e 2 H 2 O O 2 +4 H+ +4 e. Here reaction of H 2 O is preferred

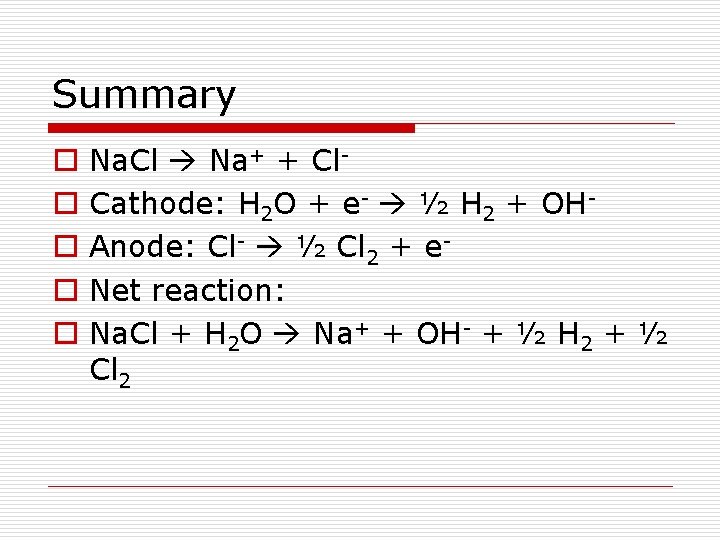
Summary o o o Na. Cl Na+ + Cl. Cathode: H 2 O + e- ½ H 2 + OHAnode: Cl- ½ Cl 2 + e. Net reaction: Na. Cl + H 2 O Na+ + OH- + ½ H 2 + ½ Cl 2

Batteries o Battery is a galvanic cell in which chemical energy of the redox reaction is converted into electrical energy. o Mainly two types: o Primary batteries o Secondary batteries

Primary Batteries o In primary batteries, reaction occurs only once. o After use over a period of time, these become dead and cannot be reused. o Examples: Dry cell (or Lechlanche cell), Mercury cell

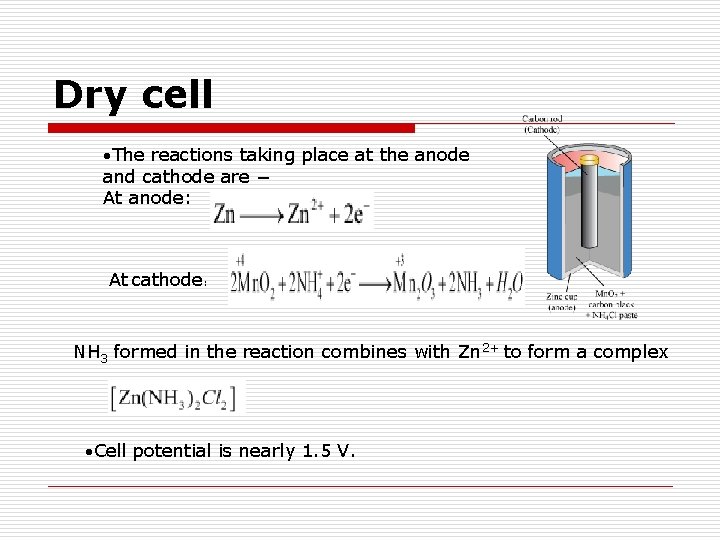
Dry cell The reactions taking place at the anode and cathode are − At anode: At cathode: NH 3 formed in the reaction combines with Zn 2+ to form a complex Cell potential is nearly 1. 5 V.

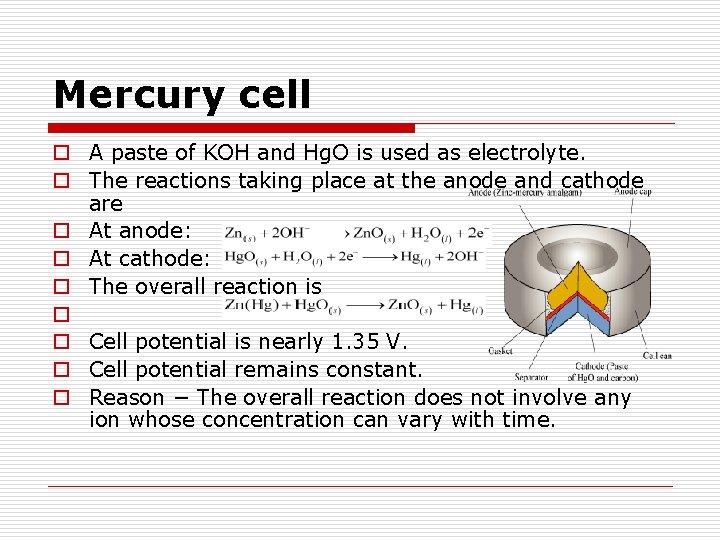
Mercury cell o A paste of KOH and Hg. O is used as electrolyte. o The reactions taking place at the anode and cathode are o At anode: o At cathode: o The overall reaction is o o Cell potential is nearly 1. 35 V. o Cell potential remains constant. o Reason − The overall reaction does not involve any ion whose concentration can vary with time.

Secondary Batteries o Secondary batteries can be recharged again by passing current through them in the opposite direction. o Examples: Lead storage battery, Nickel-cadmium cell

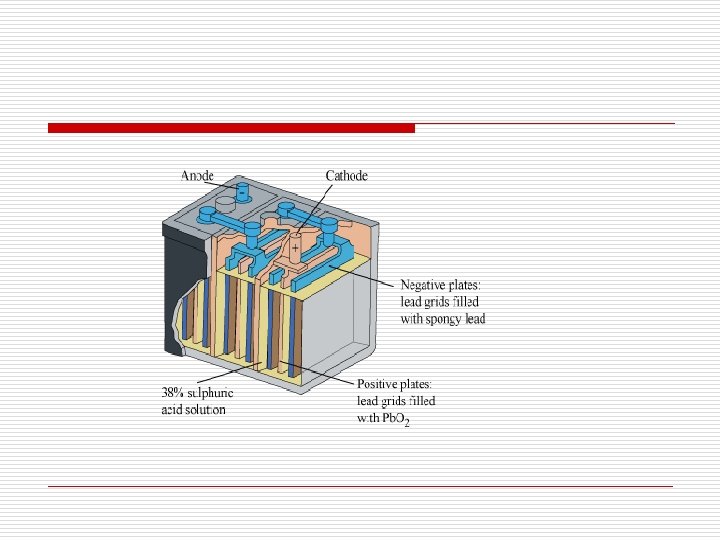
Lead storage battery o Commonly used in automobiles and invertors o Anode → Lead o Cathode is a grid of lead packed with Pb. O 2 o Electrolyte is 38% solution of H 2 SO 4


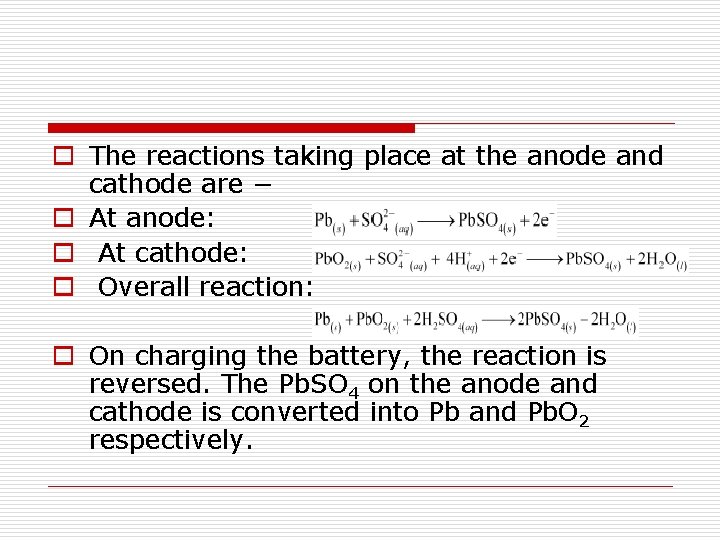
o The reactions taking place at the anode and cathode are − o At anode: o At cathode: o Overall reaction: o On charging the battery, the reaction is reversed. The Pb. SO 4 on the anode and cathode is converted into Pb and Pb. O 2 respectively.

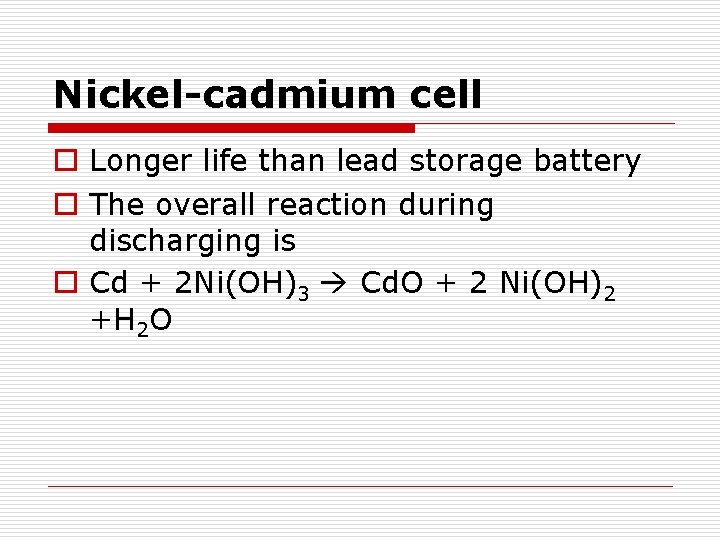
Nickel-cadmium cell o Longer life than lead storage battery o The overall reaction during discharging is o Cd + 2 Ni(OH)3 Cd. O + 2 Ni(OH)2 +H 2 O

Fuel Cells o Fuel cells are galvanic cells in which the energy of combustion of fuels is directly converted into electrical energy. o Fuels used are hydrogen, methane, methanol, etc.

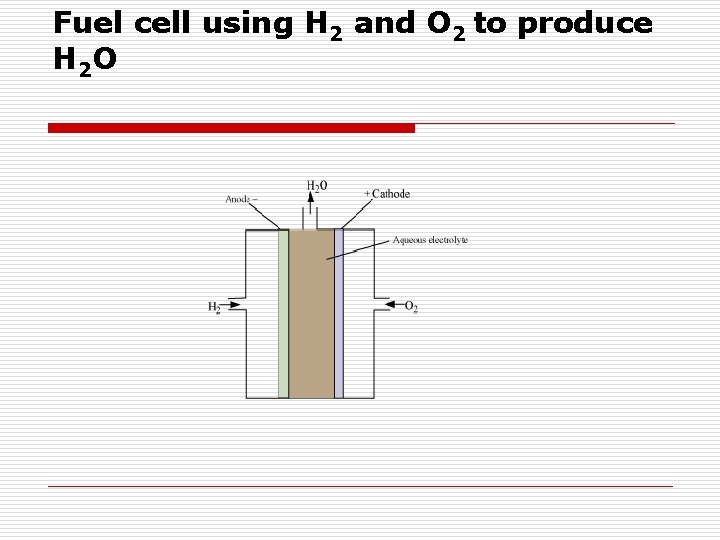
Fuel cell using H 2 and O 2 to produce H 2 O

o o o The electrode cell reactions − At anode: 2 H 2(g) + 4 OH−(aq) → 4 H 2 O(l) + 4 e− At cathode: O 2(g) + 2 H 2 O(l) + 4 e− → 4 OH−(aq) The overall reaction is 2 H 2(g) + O 2(g) → 2 H 2 O(l)

Corrosion o It is the process of slow conversion of metals into their oxides (undesirable) by the action of moisture, oxygen and other gases of the atmosphere. o Certain metals (except the least reactive metals like Au, Pt, Pd) undergo corrosion and become coated with their oxides and other salts of the metals. o Examples: o Rusting of iron o Tarnishing of copper o Development of a green coating on copper and bronze o In the process of corrosion, metals lose electrons to oxygen and get oxidised.

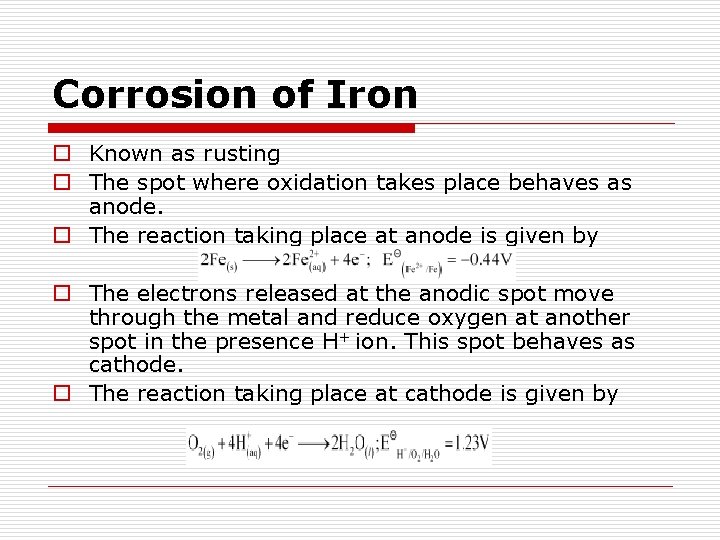
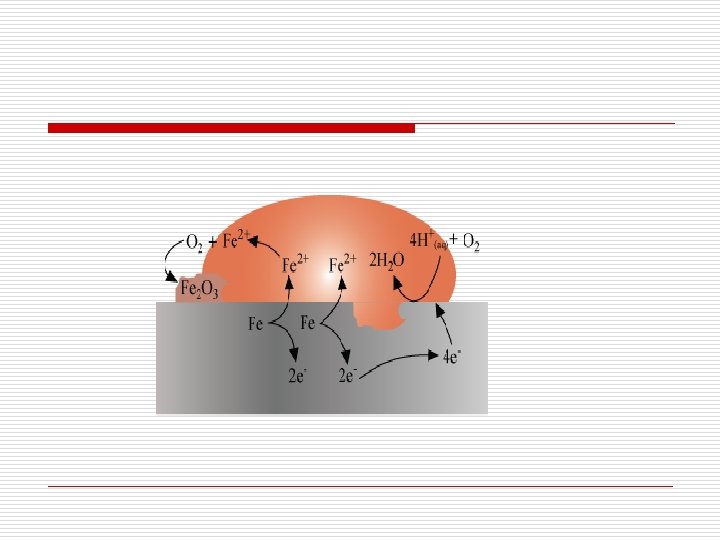
Corrosion of Iron o Known as rusting o The spot where oxidation takes place behaves as anode. o The reaction taking place at anode is given by o The electrons released at the anodic spot move through the metal and reduce oxygen at another spot in the presence H+ ion. This spot behaves as cathode. o The reaction taking place at cathode is given by

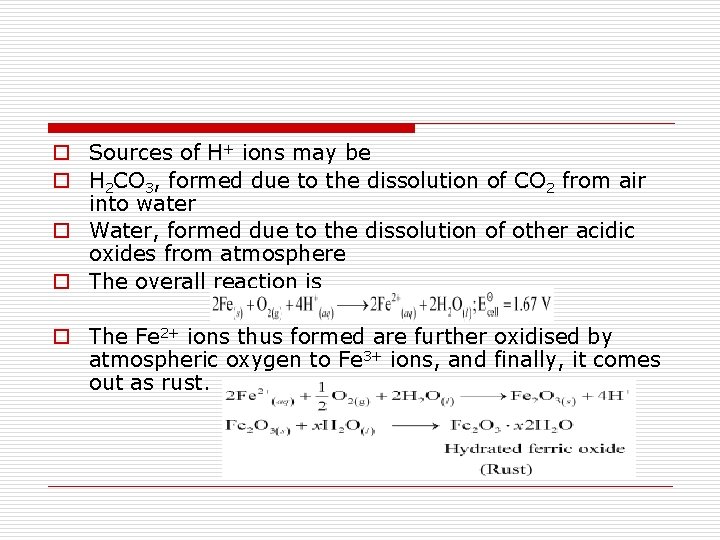
o Sources of H+ ions may be o H 2 CO 3, formed due to the dissolution of CO 2 from air into water o Water, formed due to the dissolution of other acidic oxides from atmosphere o The overall reaction is o The Fe 2+ ions thus formed are further oxidised by atmospheric oxygen to Fe 3+ ions, and finally, it comes out as rust.


Prevention of Corrosion o Preventing the surface of the metal from coming in contact with atmosphere o By covering the surface with paint or chemicals such as bis-phenol o By covering the surface with other metals such as Sn, Zn, Mg.