Electrochemistry and Redox Reactions Electrochemical processes are oxidationreduction
- Slides: 22

Electrochemistry and Redox Reactions

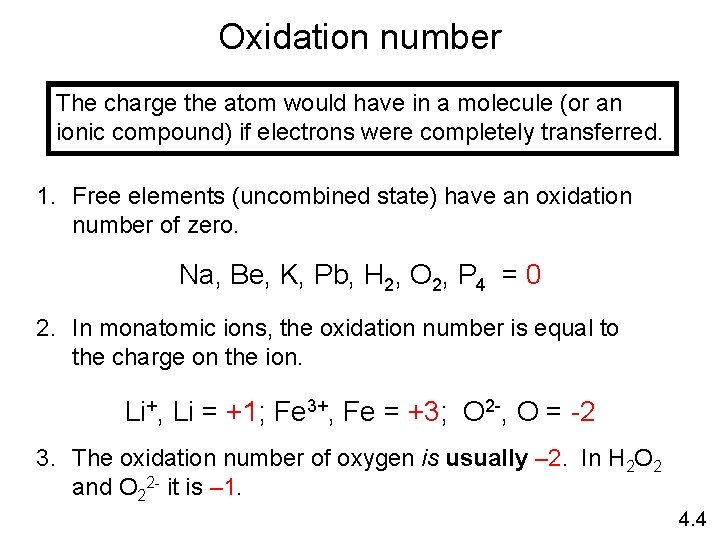
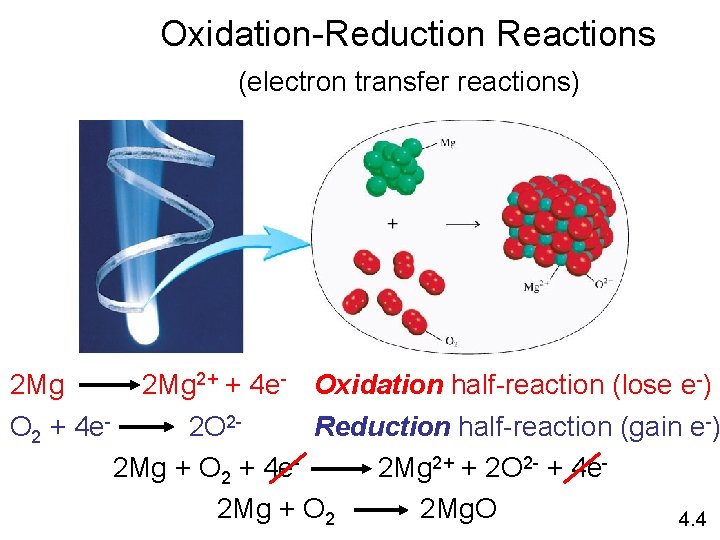
Electrochemical processes are oxidation-reduction reactions in which: • the energy released by a spontaneous reaction is converted to electricity or • electrical energy is used to cause a nonspontaneous reaction to occur 0 0 2+ 2 - 2 Mg (s) + O 2 (g) 2 Mg O 2 + 4 e- 2 Mg. O (s) 2 Mg 2+ + 4 e- Oxidation half-reaction (lose e-) 2 O 2 - Reduction half-reaction (gain e-) 19. 1

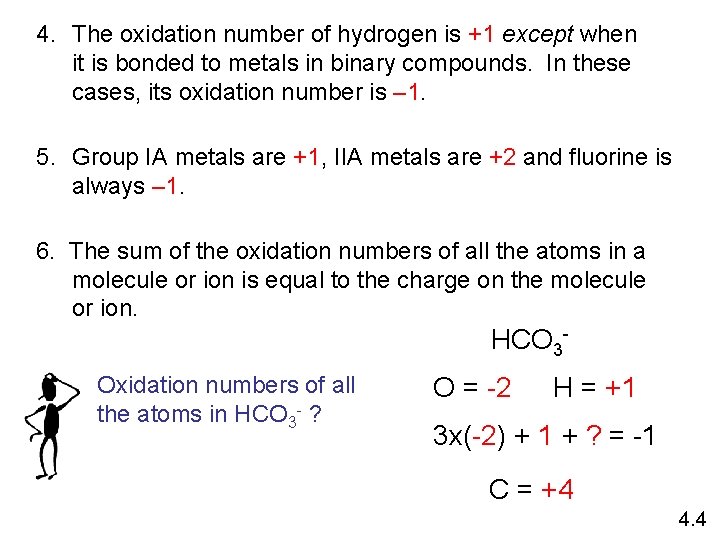
Oxidation number The charge the atom would have in a molecule (or an ionic compound) if electrons were completely transferred. 1. Free elements (uncombined state) have an oxidation number of zero. Na, Be, K, Pb, H 2, O 2, P 4 = 0 2. In monatomic ions, the oxidation number is equal to the charge on the ion. Li+, Li = +1; Fe 3+, Fe = +3; O 2 -, O = -2 3. The oxidation number of oxygen is usually – 2. In H 2 O 2 and O 22 - it is – 1. 4. 4

4. The oxidation number of hydrogen is +1 except when it is bonded to metals in binary compounds. In these cases, its oxidation number is – 1. 5. Group IA metals are +1, IIA metals are +2 and fluorine is always – 1. 6. The sum of the oxidation numbers of all the atoms in a molecule or ion is equal to the charge on the molecule or ion. HCO 3 Oxidation numbers of all the atoms in HCO 3 - ? O = -2 H = +1 3 x(-2) + 1 + ? = -1 C = +4 4. 4

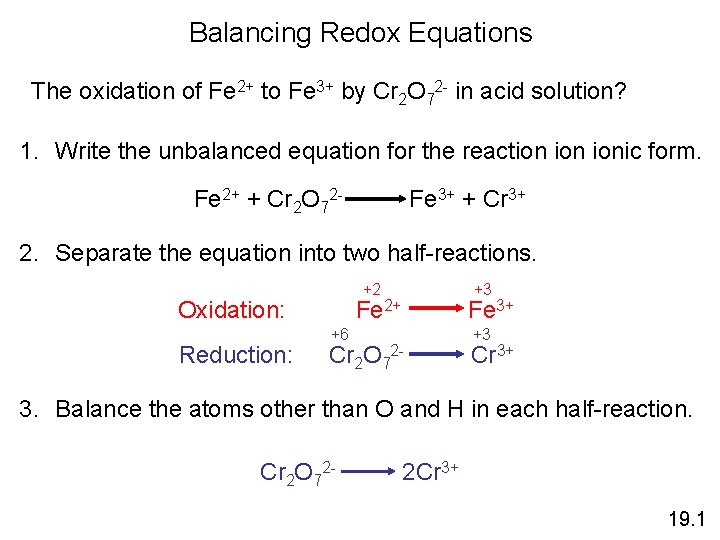
Balancing Redox Equations The oxidation of Fe 2+ to Fe 3+ by Cr 2 O 72 - in acid solution? 1. Write the unbalanced equation for the reaction ionic form. Fe 2+ + Cr 2 O 72 - Fe 3+ + Cr 3+ 2. Separate the equation into two half-reactions. +2 Oxidation: Reduction: +3 Fe 2+ +6 Cr 2 O 7 Fe 3+ 2 - +3 Cr 3+ 3. Balance the atoms other than O and H in each half-reaction. Cr 2 O 72 - 2 Cr 3+ 19. 1

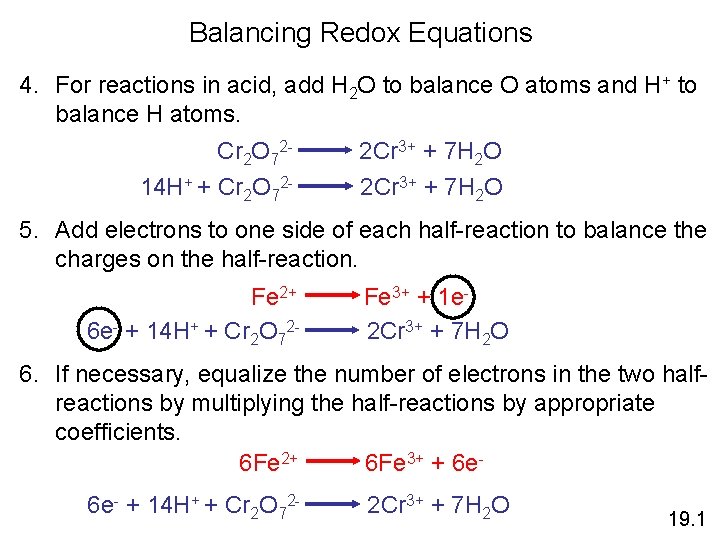
Balancing Redox Equations 4. For reactions in acid, add H 2 O to balance O atoms and H+ to balance H atoms. Cr 2 O 7214 H+ + Cr 2 O 72 - 2 Cr 3+ + 7 H 2 O 5. Add electrons to one side of each half-reaction to balance the charges on the half-reaction. Fe 2+ 6 e- + 14 H+ + Cr 2 O 72 - Fe 3+ + 1 e 2 Cr 3+ + 7 H 2 O 6. If necessary, equalize the number of electrons in the two halfreactions by multiplying the half-reactions by appropriate coefficients. 6 Fe 2+ 6 Fe 3+ + 6 e 6 e- + 14 H+ + Cr 2 O 72 - 2 Cr 3+ + 7 H 2 O 19. 1

Oxidation-Reduction Reactions (electron transfer reactions) 2 Mg 2+ + 4 e- Oxidation half-reaction (lose e-) O 2 + 4 e 2 O 2 Reduction half-reaction (gain e-) 2 Mg + O 2 + 4 e 2 Mg 2+ + 2 O 2 - + 4 e 2 Mg + O 2 2 Mg. O 4. 4

4. 4

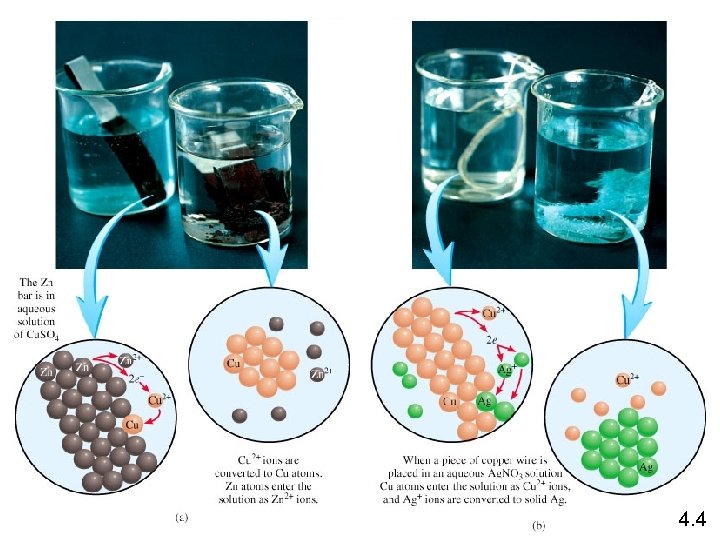
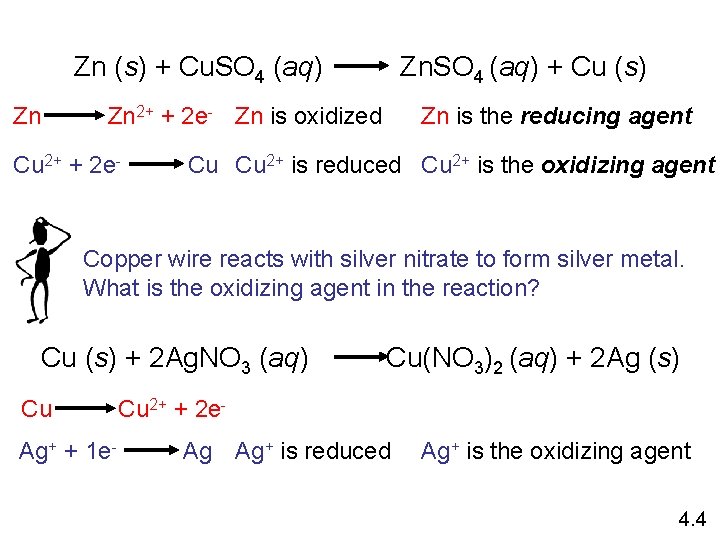
Zn (s) + Cu. SO 4 (aq) Zn Zn. SO 4 (aq) + Cu (s) Zn is the reducing agent Zn 2+ + 2 e- Zn is oxidized Cu 2+ + 2 e- Cu Cu 2+ is reduced Cu 2+ is the oxidizing agent Copper wire reacts with silver nitrate to form silver metal. What is the oxidizing agent in the reaction? Cu (s) + 2 Ag. NO 3 (aq) Cu Ag+ + 1 e- Cu(NO 3)2 (aq) + 2 Ag (s) Cu 2+ + 2 e. Ag Ag+ is reduced Ag+ is the oxidizing agent 4. 4

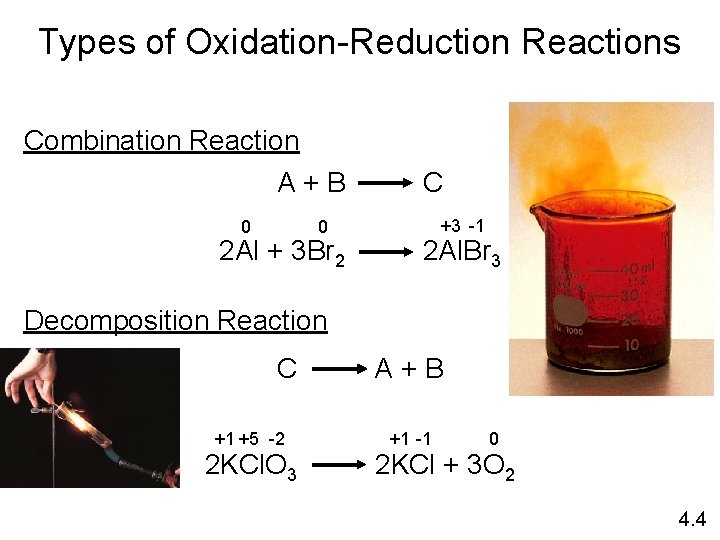
Types of Oxidation-Reduction Reactions Combination Reaction A+B 0 0 2 Al + 3 Br 2 C +3 -1 2 Al. Br 3 Decomposition Reaction C +1 +5 -2 2 KCl. O 3 A+B +1 -1 0 2 KCl + 3 O 2 4. 4

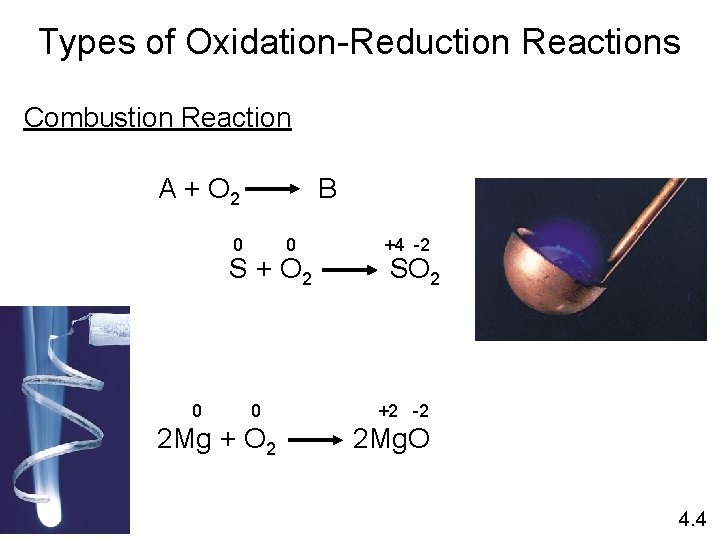
Types of Oxidation-Reduction Reactions Combustion Reaction A + O 2 B 0 0 S + O 2 0 0 2 Mg + O 2 +4 -2 SO 2 +2 -2 2 Mg. O 4. 4

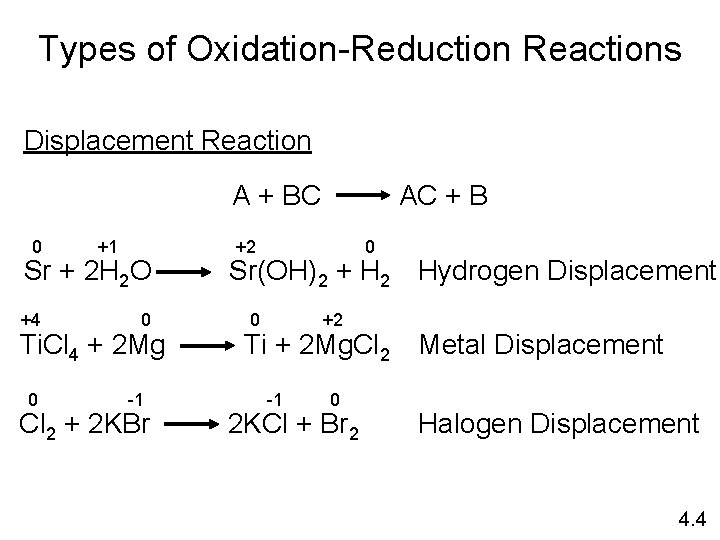
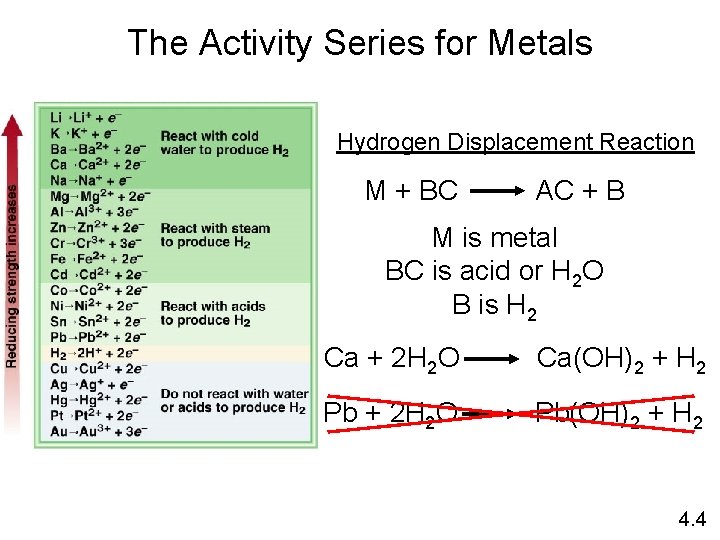
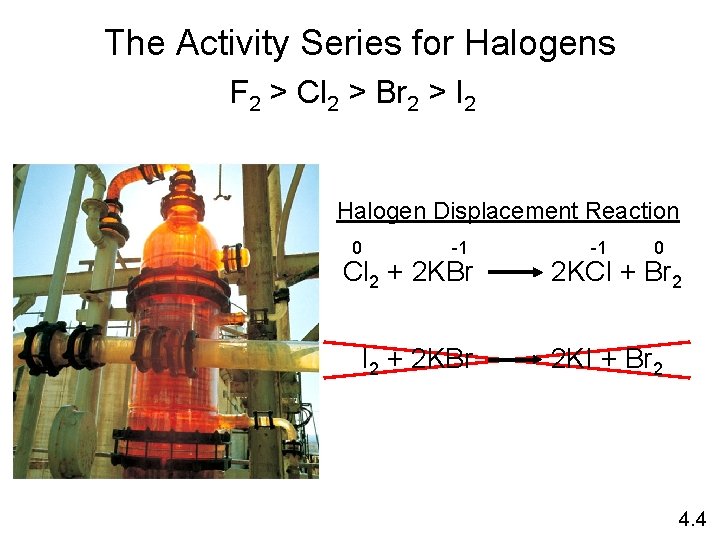
Types of Oxidation-Reduction Reactions Displacement Reaction A + BC 0 +1 Sr + 2 H 2 O +4 0 Ti. Cl 4 + 2 Mg 0 -1 Cl 2 + 2 KBr AC + B +2 0 Sr(OH)2 + H 2 Hydrogen Displacement 0 +2 Ti + 2 Mg. Cl 2 Metal Displacement -1 0 2 KCl + Br 2 Halogen Displacement 4. 4

The Activity Series for Metals Hydrogen Displacement Reaction M + BC AC + B M is metal BC is acid or H 2 O B is H 2 Ca + 2 H 2 O Ca(OH)2 + H 2 Pb + 2 H 2 O Pb(OH)2 + H 2 4. 4

The Activity Series for Halogens F 2 > Cl 2 > Br 2 > I 2 Halogen Displacement Reaction 0 -1 Cl 2 + 2 KBr I 2 + 2 KBr -1 0 2 KCl + Br 2 2 KI + Br 2 4. 4

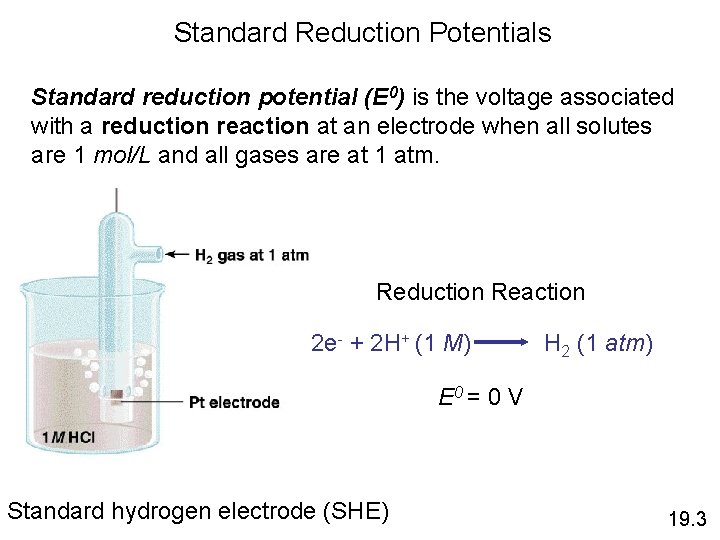
Standard Reduction Potentials Standard reduction potential (E 0) is the voltage associated with a reduction reaction at an electrode when all solutes are 1 mol/L and all gases are at 1 atm. Reduction Reaction 2 e- + 2 H+ (1 M) H 2 (1 atm) E 0 = 0 V Standard hydrogen electrode (SHE) 19. 3

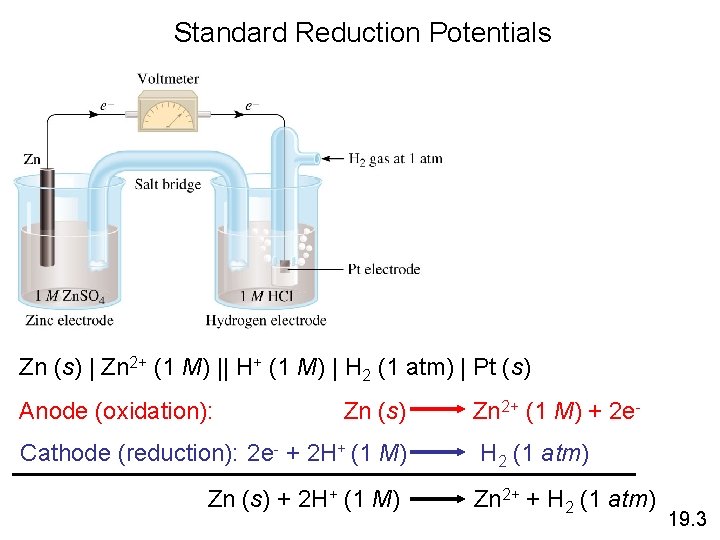
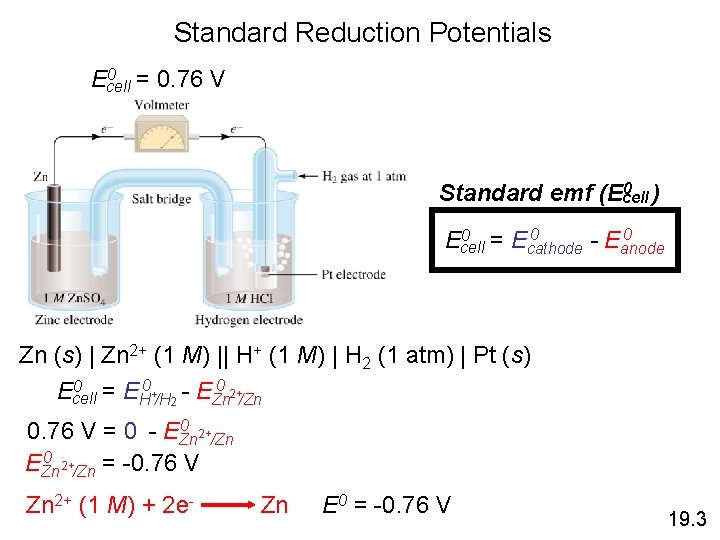
Standard Reduction Potentials Zn (s) | Zn 2+ (1 M) || H+ (1 M) | H 2 (1 atm) | Pt (s) Anode (oxidation): Zn (s) Cathode (reduction): 2 e- + 2 H+ (1 M) Zn (s) + 2 H+ (1 M) Zn 2+ (1 M) + 2 e. H 2 (1 atm) Zn 2+ + H 2 (1 atm) 19. 3

Standard Reduction Potentials 0 = 0. 76 V Ecell 0 ) Standard emf (Ecell 0 0 = E 0 Ecell cathode - Eanode Zn (s) | Zn 2+ (1 M) || H+ (1 M) | H 2 (1 atm) | Pt (s) 0 = E 0 + - E 0 2+ Ecell H /H 2 Zn /Zn 0 2+ 0. 76 V = 0 - EZn /Zn 0 2+ EZn /Zn = -0. 76 V Zn 2+ (1 M) + 2 e- Zn E 0 = -0. 76 V 19. 3

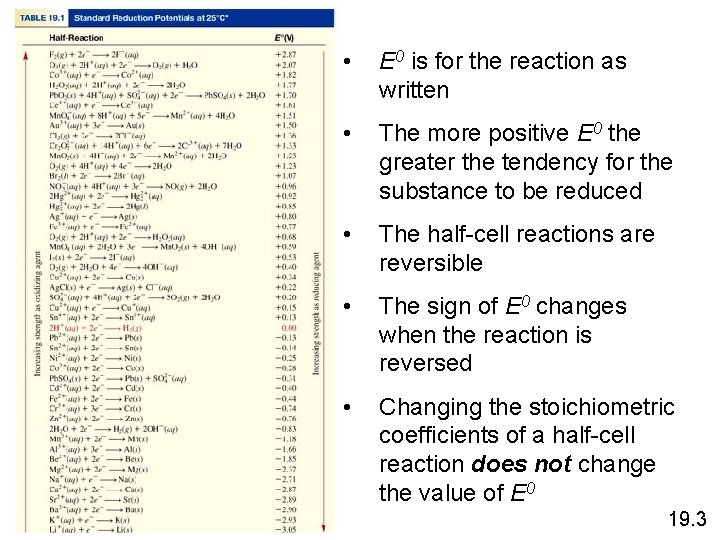
• E 0 is for the reaction as written • The more positive E 0 the greater the tendency for the substance to be reduced • The half-cell reactions are reversible • The sign of E 0 changes when the reaction is reversed • Changing the stoichiometric coefficients of a half-cell reaction does not change the value of E 0 19. 3

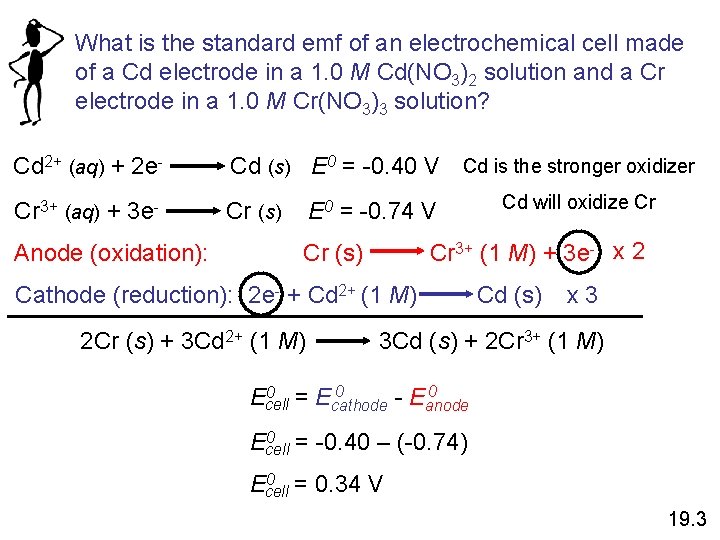
What is the standard emf of an electrochemical cell made of a Cd electrode in a 1. 0 M Cd(NO 3)2 solution and a Cr electrode in a 1. 0 M Cr(NO 3)3 solution? Cd 2+ (aq) + 2 e- Cd (s) E 0 = -0. 40 V Cd is the stronger oxidizer Cr 3+ (aq) + 3 e- Cr (s) Anode (oxidation): E 0 = -0. 74 V Cr 3+ (1 M) + 3 e- x 2 Cr (s) Cathode (reduction): 2 e- + Cd 2+ (1 M) 2 Cr (s) + 3 Cd 2+ (1 M) Cd will oxidize Cr Cd (s) x 3 3 Cd (s) + 2 Cr 3+ (1 M) 0 0 = E 0 Ecell cathode - Eanode 0 = -0. 40 – (-0. 74) Ecell 0 = 0. 34 V Ecell 19. 3

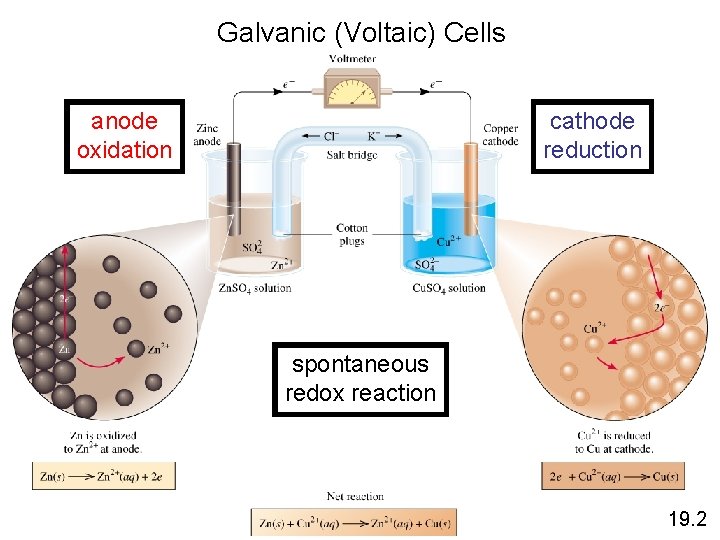
Galvanic (Voltaic) Cells anode oxidation cathode reduction spontaneous redox reaction 19. 2

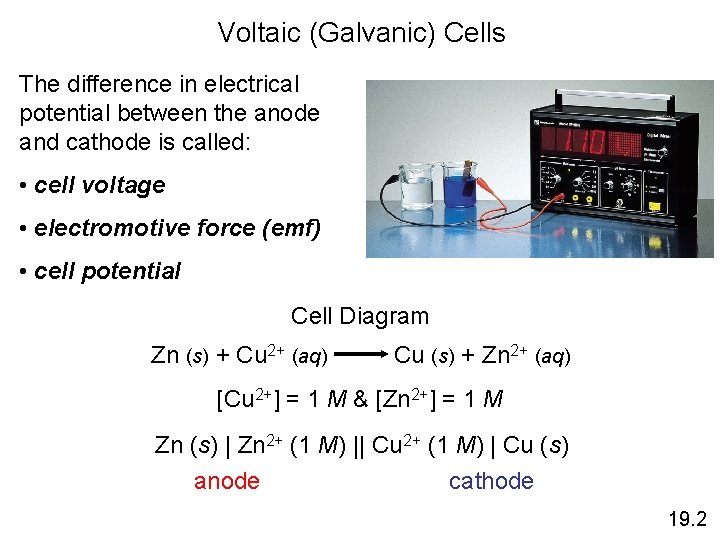
Voltaic (Galvanic) Cells The difference in electrical potential between the anode and cathode is called: • cell voltage • electromotive force (emf) • cell potential Cell Diagram Zn (s) + Cu 2+ (aq) Cu (s) + Zn 2+ (aq) [Cu 2+] = 1 M & [Zn 2+] = 1 M Zn (s) | Zn 2+ (1 M) || Cu 2+ (1 M) | Cu (s) anode cathode 19. 2

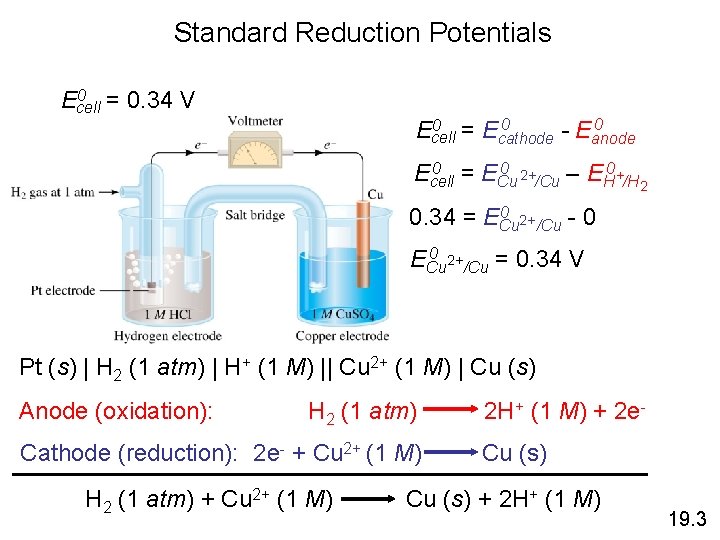
Standard Reduction Potentials 0 = 0. 34 V Ecell 0 0 = E 0 Ecell cathode - Eanode 0 = E 0 2+ 0 Ecell Cu /Cu – EH +/H 2 0 2+ 0. 34 = ECu /Cu - 0 0 2+ ECu /Cu = 0. 34 V Pt (s) | H 2 (1 atm) | H+ (1 M) || Cu 2+ (1 M) | Cu (s) Anode (oxidation): H 2 (1 atm) Cathode (reduction): 2 e- + Cu 2+ (1 M) H 2 (1 atm) + Cu 2+ (1 M) 2 H+ (1 M) + 2 e. Cu (s) + 2 H+ (1 M) 19. 3