ELECTROCHEMICAL SURFACE FINISHING OF ADDITIVELY MANUFACTURED PARTS H



- Slides: 22

ELECTROCHEMICAL SURFACE FINISHING OF ADDITIVELY MANUFACTURED PARTS H. Garich 1, T. Hall 1, E. J. Taylor 1, D. Lui 1, J. Porter 2, and B. Hayes 2 1 Faraday Technology (Dayton, OH) 2 UES, Inc. (Dayton, OH) 233 rd ECS Meeting, Seattle, WA USA May 16, 2018

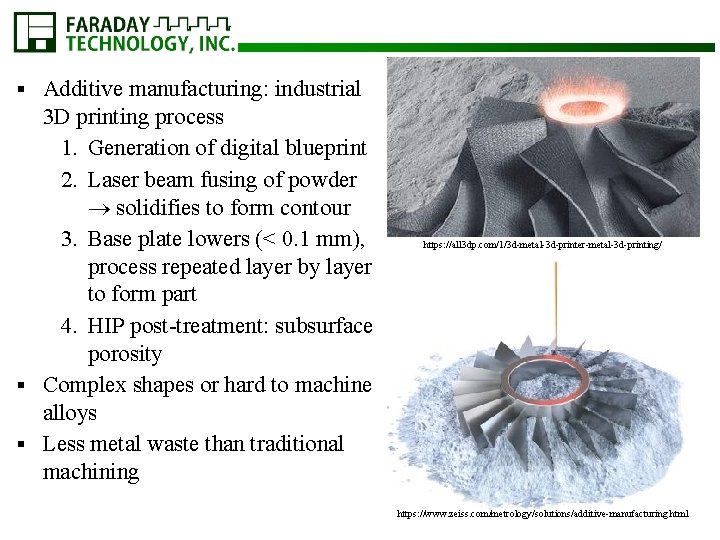
Additive manufacturing: industrial 3 D printing process 1. Generation of digital blueprint 2. Laser beam fusing of powder solidifies to form contour 3. Base plate lowers (< 0. 1 mm), process repeated layer by layer to form part 4. HIP post-treatment: subsurface porosity § Complex shapes or hard to machine alloys § Less metal waste than traditional machining § https: //all 3 dp. com/1/3 d-metal-3 d-printer-metal-3 d-printing/ https: //www. zeiss. com/metrology/solutions/additive-manufacturing. html

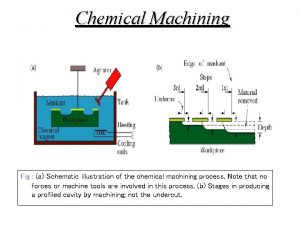
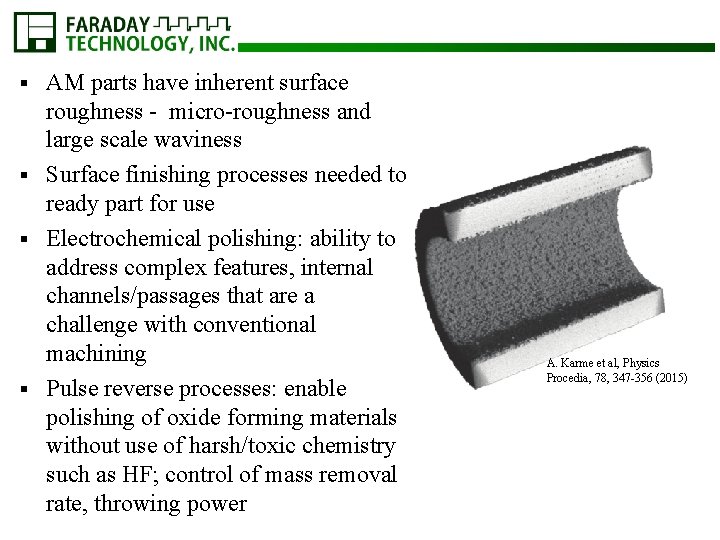
AM parts have inherent surface roughness - micro-roughness and large scale waviness § Surface finishing processes needed to ready part for use § Electrochemical polishing: ability to address complex features, internal channels/passages that are a challenge with conventional machining § Pulse reverse processes: enable polishing of oxide forming materials without use of harsh/toxic chemistry such as HF; control of mass removal rate, throwing power § A. Karme et al, Physics Procedia, 78, 347 -356 (2015)

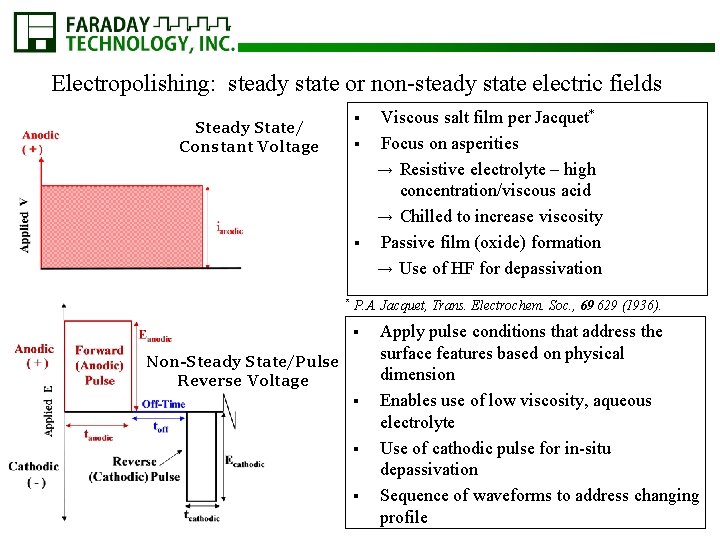
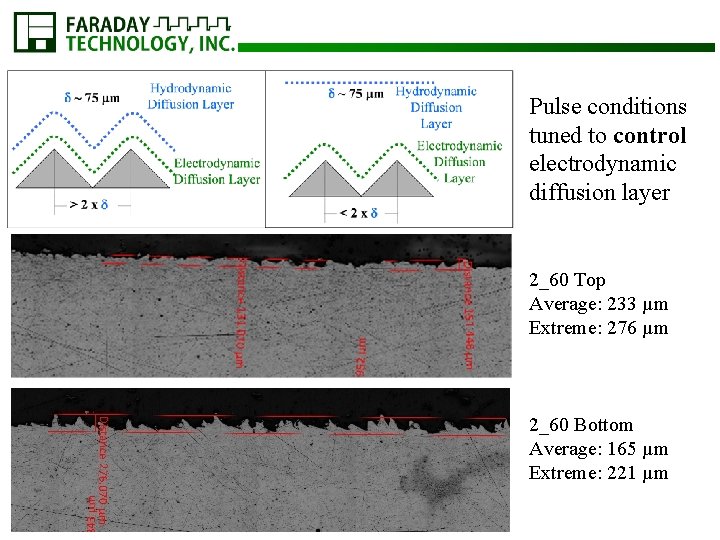
Electropolishing: steady state or non-steady state electric fields Viscous salt film per Jacquet* § Focus on asperities → Resistive electrolyte – high concentration/viscous acid → Chilled to increase viscosity § Passive film (oxide) formation → Use of HF for depassivation § Steady State/ Constant Voltage * P. A. Jacquet, Trans. Electrochem. Soc. , 69 629 (1936). § Non-Steady State/Pulse Reverse Voltage § § § Apply pulse conditions that address the surface features based on physical dimension Enables use of low viscosity, aqueous electrolyte Use of cathodic pulse for in-situ depassivation Sequence of waveforms to address changing profile

Pulse conditions tuned to control electrodynamic diffusion layer 2_60 Top Average: 233 µm Extreme: 276 µm 2_60 Bottom Average: 165 µm Extreme: 221 µm

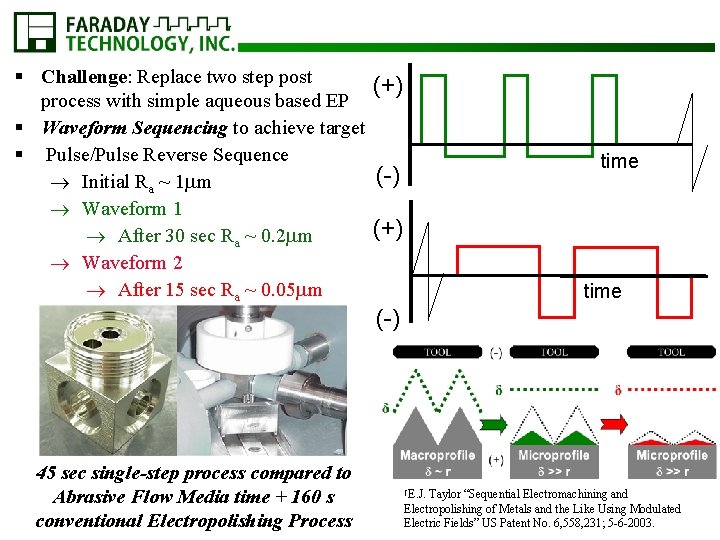
§ Challenge: Replace two step post (+) process with simple aqueous based EP § Waveform Sequencing to achieve target § Pulse/Pulse Reverse Sequence (-) Initial Ra ~ 1 m Waveform 1 (+) After 30 sec Ra ~ 0. 2 m Waveform 2 After 15 sec Ra ~ 0. 05 m time (-) 45 sec single-step process compared to Abrasive Flow Media time + 160 s conventional Electropolishing Process †E. J. Taylor “Sequential Electromachining and Electropolishing of Metals and the Like Using Modulated Electric Fields” US Patent No. 6, 558, 231; 5 -6 -2003.

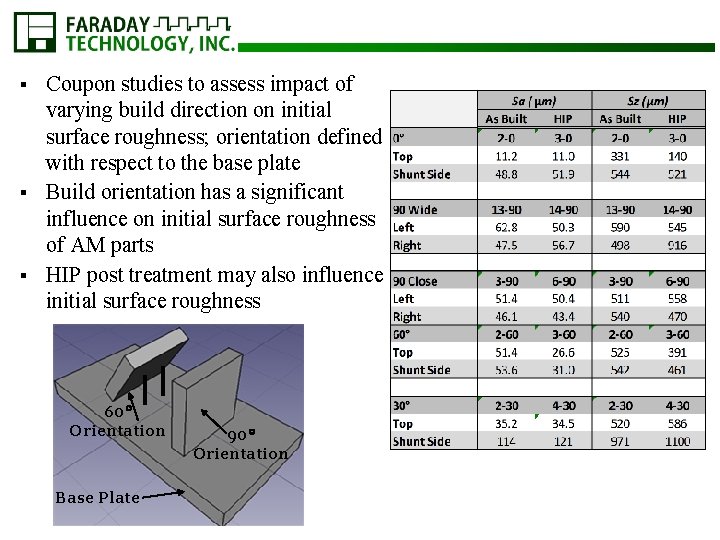
Coupon studies to assess impact of varying build direction on initial surface roughness; orientation defined with respect to the base plate § Build orientation has a significant influence on initial surface roughness of AM parts § HIP post treatment may also influence initial surface roughness § 60 Orientation Base Plate 90 Orientation

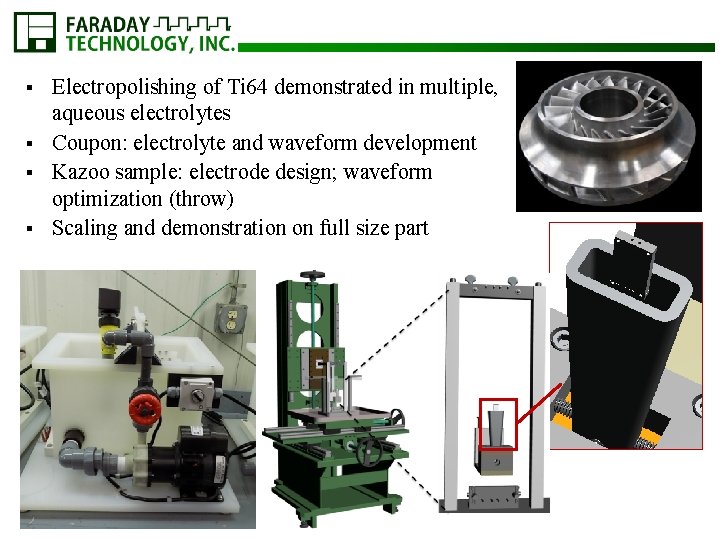
Electropolishing of Ti 64 demonstrated in multiple, aqueous electrolytes § Coupon: electrolyte and waveform development § Kazoo sample: electrode design; waveform optimization (throw) § Scaling and demonstration on full size part §

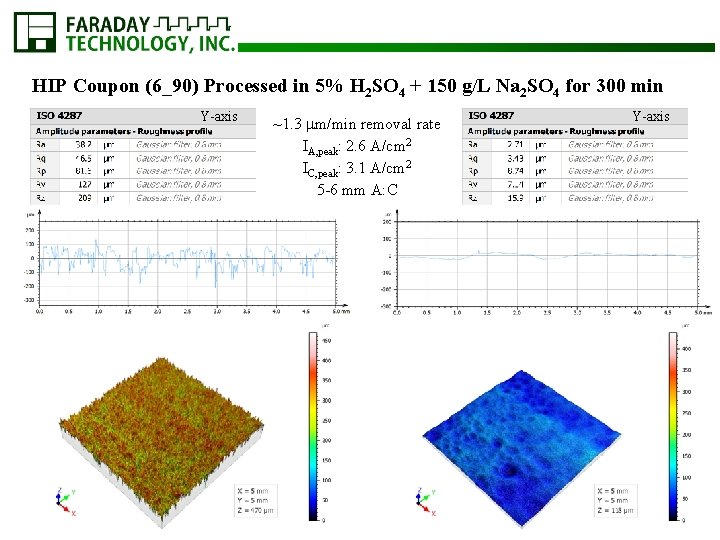
HIP Coupon (6_90) Processed in 5% H 2 SO 4 + 150 g/L Na 2 SO 4 for 300 min Y-axis ~1. 3 m/min removal rate IA, peak: 2. 6 A/cm 2 IC, peak: 3. 1 A/cm 2 5 -6 mm A: C Y-axis

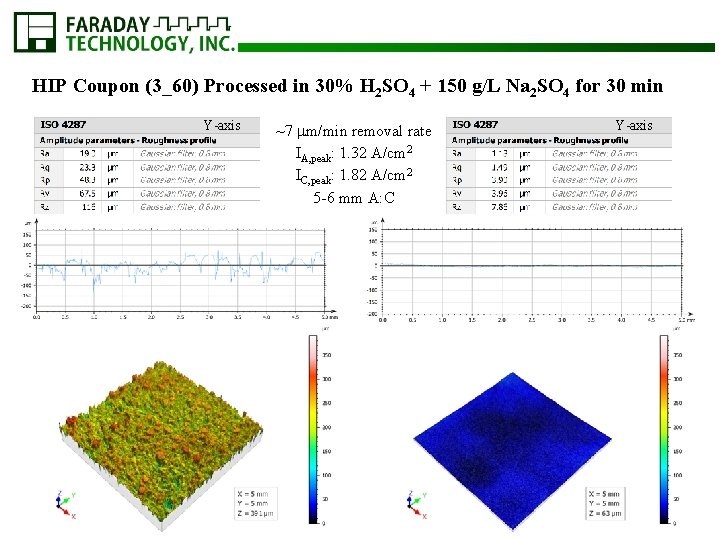
HIP Coupon (3_60) Processed in 30% H 2 SO 4 + 150 g/L Na 2 SO 4 for 30 min Y-axis ~7 m/min removal rate IA, peak: 1. 32 A/cm 2 IC, peak: 1. 82 A/cm 2 5 -6 mm A: C Y-axis

Summary of Tests in Sulfuric Acid Electrolytes In 5% (w/w) H 2 SO 4 + 150 g/L Na 2 SO 4: → Issues associated with smutting and slow removal rates → Low waveform frequencies and lower applied voltage to smooth rough surfaces → Waveform must be changed to address changing surface profile § In 30% (w/w) H 2 SO 4 + 150 g/L Na 2 SO 4: → Less luster than observed with 5% electrolyte → Issues of smutting are minimized → Ra decreases rapidly with less material losses → Cathodic pulse must be balanced with other parameters to ensure oxide removal and subsequent material removal § The high current densities observed with these electrolytes are prohibitive for scaling activities when decreasing anode-to-cathode spacing→ evaluate lower conductivity electrolyte §

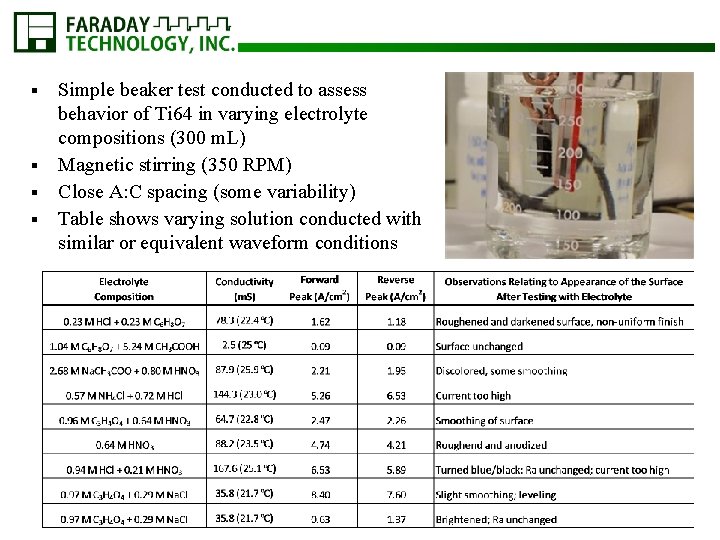
Simple beaker test conducted to assess behavior of Ti 64 in varying electrolyte compositions (300 m. L) § Magnetic stirring (350 RPM) § Close A: C spacing (some variability) § Table shows varying solution conducted with similar or equivalent waveform conditions §

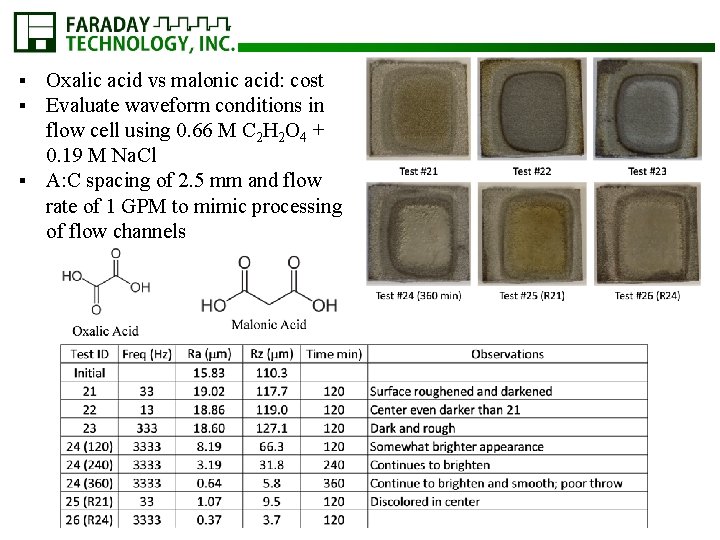
Oxalic acid vs malonic acid: cost Evaluate waveform conditions in flow cell using 0. 66 M C 2 H 2 O 4 + 0. 19 M Na. Cl § A: C spacing of 2. 5 mm and flow rate of 1 GPM to mimic processing of flow channels § §

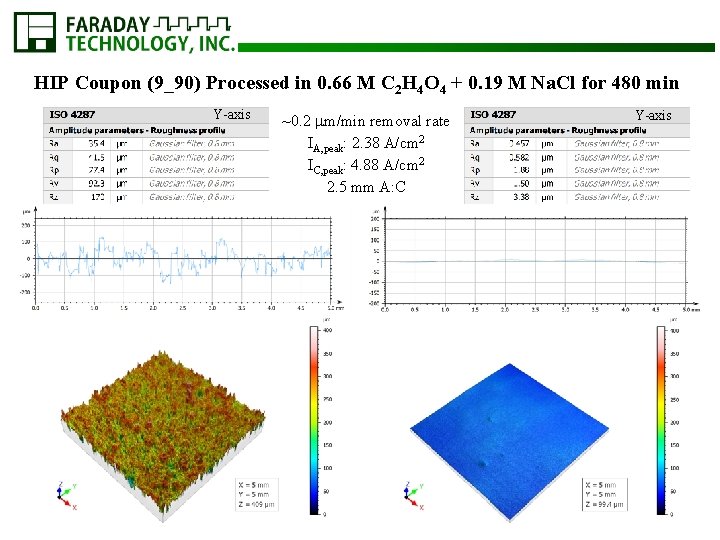
HIP Coupon (9_90) Processed in 0. 66 M C 2 H 4 O 4 + 0. 19 M Na. Cl for 480 min Y-axis ~0. 2 m/min removal rate IA, peak: 2. 38 A/cm 2 IC, peak: 4. 88 A/cm 2 2. 5 mm A: C Y-axis

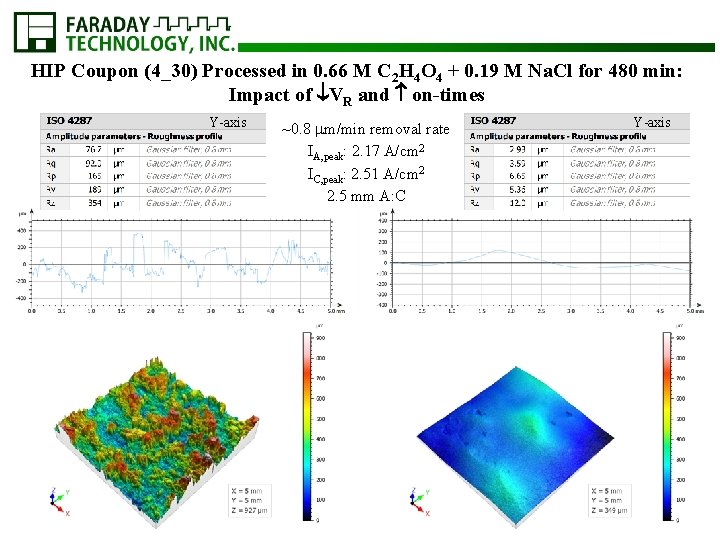
HIP Coupon (4_30) Processed in 0. 66 M C 2 H 4 O 4 + 0. 19 M Na. Cl for 480 min: Impact of VR and on-times Y-axis ~0. 8 m/min removal rate IA, peak: 2. 17 A/cm 2 IC, peak: 2. 51 A/cm 2 2. 5 mm A: C Y-axis

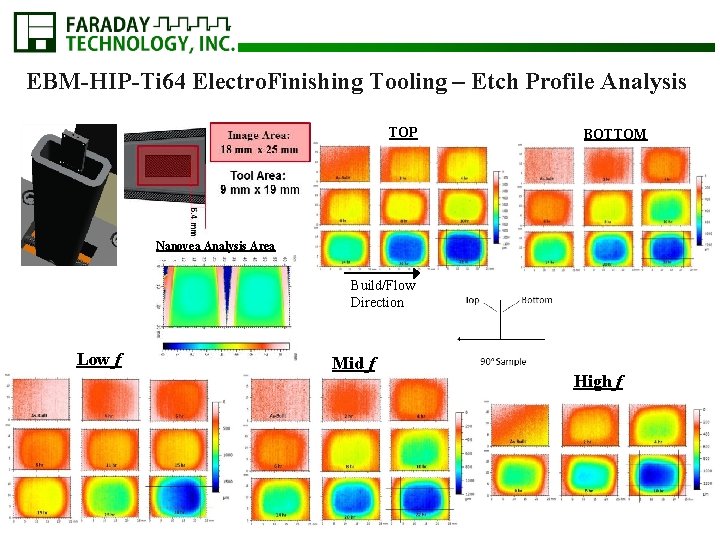
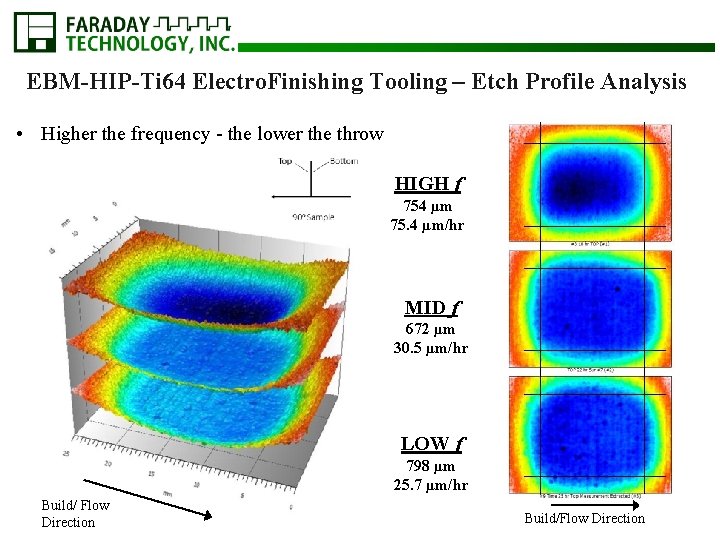
EBM-HIP-Ti 64 Electro. Finishing Tooling – Etch Profile Analysis TOP BOTTOM Nanovea Analysis Area Build/Flow Direction Low f Mid f High f

EBM-HIP-Ti 64 Electro. Finishing Tooling – Etch Profile Analysis • Higher the frequency - the lower the throw HIGH f 754 µm 75. 4 µm/hr MID f 672 µm 30. 5 µm/hr LOW f 798 µm 25. 7 µm/hr Build/ Flow Direction Build/Flow Direction

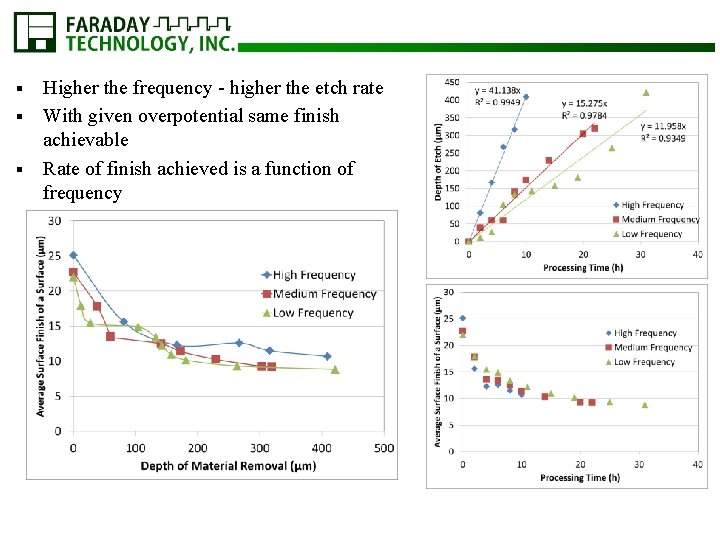
Higher the frequency - higher the etch rate § With given overpotential same finish achievable § Rate of finish achieved is a function of frequency §

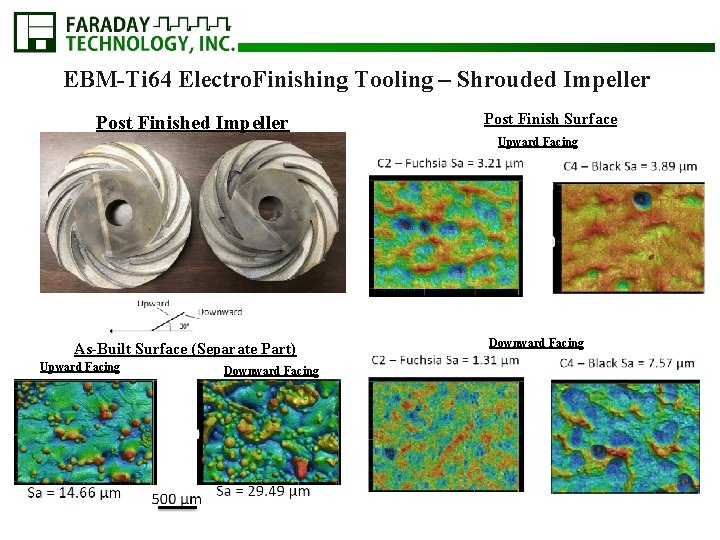
EBM-Ti 64 Electro. Finishing Tooling – Shrouded Impeller

EBM-Ti 64 Electro. Finishing Tooling – Shrouded Impeller Post Finish Surface Upward Facing As-Built Surface (Separate Part) Upward Facing Downward Facing

o Demonstrated potential to use P/PR Electro. Finishing techniques to finish various Conventionally Manufactured and Additively Manufactured materials in water based HF-free electrolytes § Ti 64, Hast-X, IN 718, etc… o Demonstrated potential to selectively remove dissimilar materials from surface feature o Demonstrated the potential of electrically controlled electrochemical processes and sequencing to produce the desired finish while limiting material removal o Applications: § Medical, Automotive, Aerospace, Energy Markets, Even Superconducting Nb structures

ACKNOWLEDGEMENTS THANK YOU FOR YOUR ATTENTION! QUESTIONS? Financial support: USAF RIF, NASA, Client Confidential Contact Information: Tim Hall, Maria Inman, or EJ Taylor Ph: 937 -836 -7749
 Additive manufacturing steps
Additive manufacturing steps Surface finishing operations in production technology
Surface finishing operations in production technology Sympathetic nervous system
Sympathetic nervous system Electrochemical impedance spectroscopy
Electrochemical impedance spectroscopy The electrochemical series table
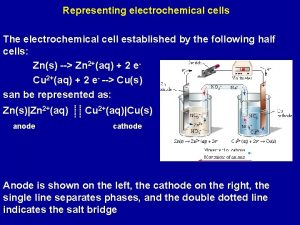
The electrochemical series table Cathode and anode
Cathode and anode Cathode vs anode equation
Cathode vs anode equation What are electrochemical series
What are electrochemical series Refractory period anatomy
Refractory period anatomy Giner electrochemical systems
Giner electrochemical systems Types of electrochemical corrosion
Types of electrochemical corrosion Khancademy
Khancademy Vapor
Vapor Melvin bae
Melvin bae Electrochemical deposition
Electrochemical deposition Electrochemical impulse
Electrochemical impulse Electrochemical series order
Electrochemical series order Electrochemical series
Electrochemical series Balance redox
Balance redox Electrochemical machining advantages and disadvantages
Electrochemical machining advantages and disadvantages Difference between dry and wet corrosion
Difference between dry and wet corrosion What is a half reaction
What is a half reaction Electrochemical theory of corrosion
Electrochemical theory of corrosion