Electrochemical prosses 4 The electrochemical processes are called





















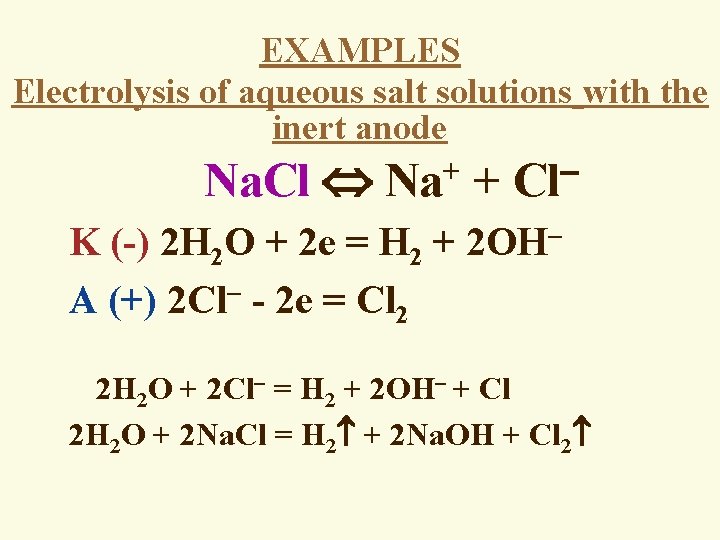
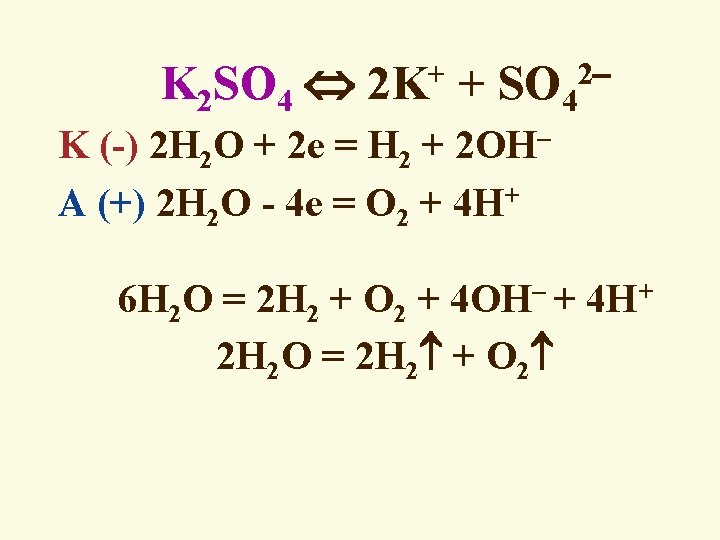
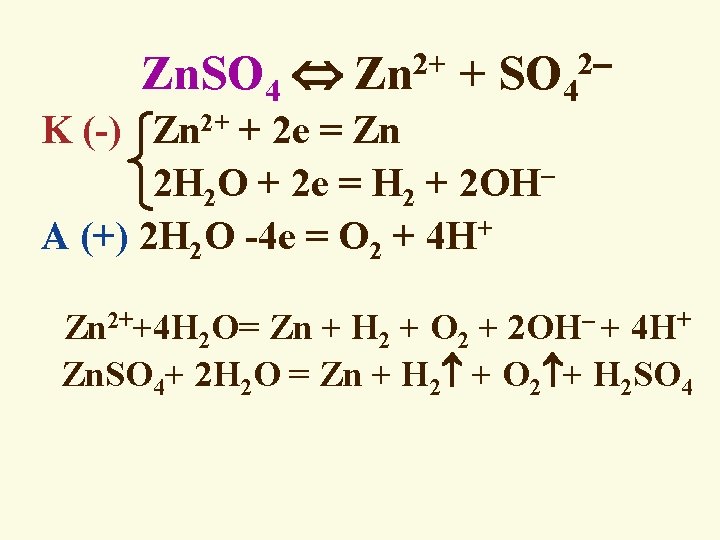
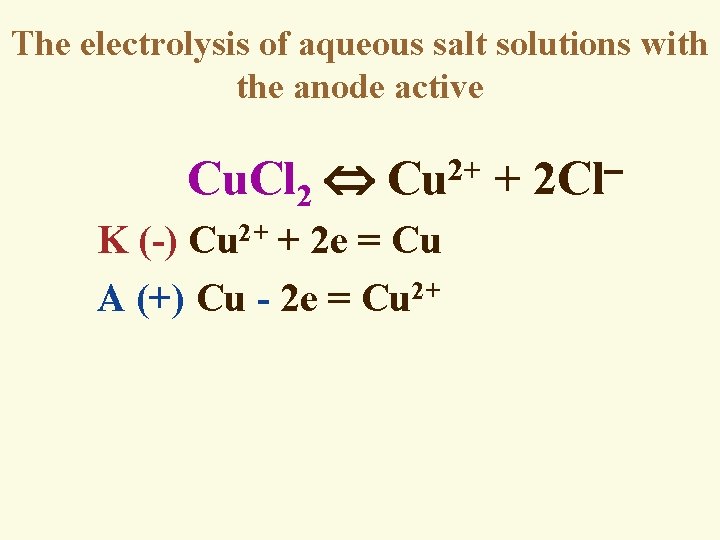

![Мeq. – molar mass of the equivalent I – electrical current [A] t - Мeq. – molar mass of the equivalent I – electrical current [A] t -](https://slidetodoc.com/presentation_image_h/2f6d474476b681d149e908dbd5c12d5b/image-62.jpg)



- Slides: 65

Electrochemical prosses

4 The electrochemical processes are called redox reactions which is accompanied by the release or absorption of electricity. 4 The electrodes are the main part of the electrochemical processes. 4 The electrode is a conductive material (metal, carbon) in contact with an electrolyte.

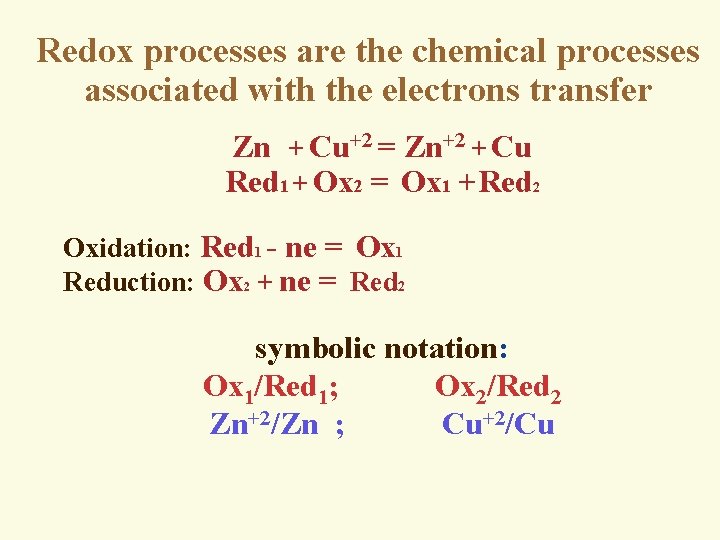
Redox processes are the chemical processes associated with the electrons transfer Zn + Cu+2 = Zn+2 + Cu Red 1 + Ox 2 = Ox 1 +Red 2 Oxidation: Red 1 - ne = Ox 1 Reduction: Ox 2 + ne = Red 2 symbolic notation: Ox 1/Red 1; Ox 2/Red 2 Zn+2/Zn ; Cu+2/Cu

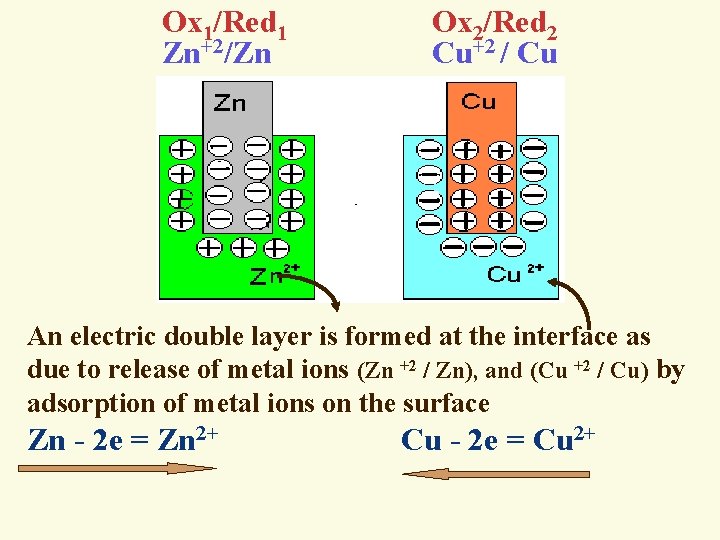
Ox 1/Red 1 Ox 2/Red 2 Zn+2/Zn Cu+2 / Cu An electric double layer is formed at the interface as due to release of metal ions (Zn +2 / Zn), and (Cu +2 / Cu) by adsorption of metal ions on the surface Zn - 2 e = Zn 2+ Cu - 2 e = Cu 2+

Equilibrium is established when the electrode potential reaches a value which is capable of stopping the oxidation (or reduction), which occurs the first time after the immersion of the metal in solution

The difference between the charges at the interface determines a potential jump 1 2

§ The magnitude of the potential difference is called an electrode potential ( ) depends on: § the nature of the electrode material § the concentrations of ions in solution § temperature § PH

Ø The absolute value of an electrode potential can not be measured Ø The electrode potential measured under standard conditions (T = 298 K, P = 1 atm, C = 1 M) with respect to another electrode called a standard electrode potential

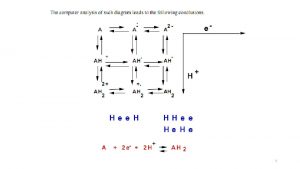
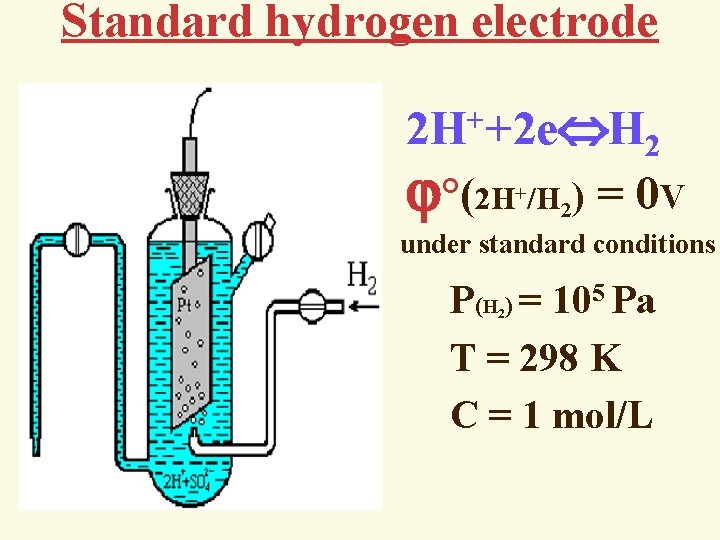
Standard hydrogen electrode + 2 H +2 e H 2 °(2 H /H ) = 0 V + 2 under standard conditions P(Н ) = 105 Pа T = 298 K C = 1 mol/L 2

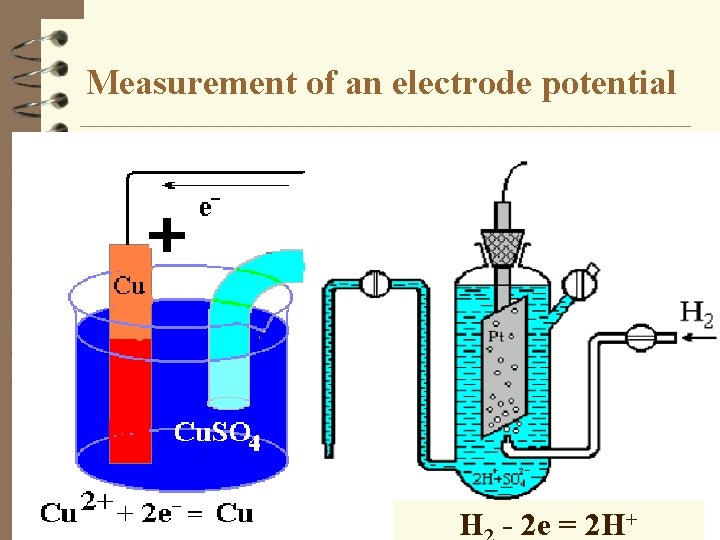
Measurement of an electrode potential Н - 2 е = 2 Н+

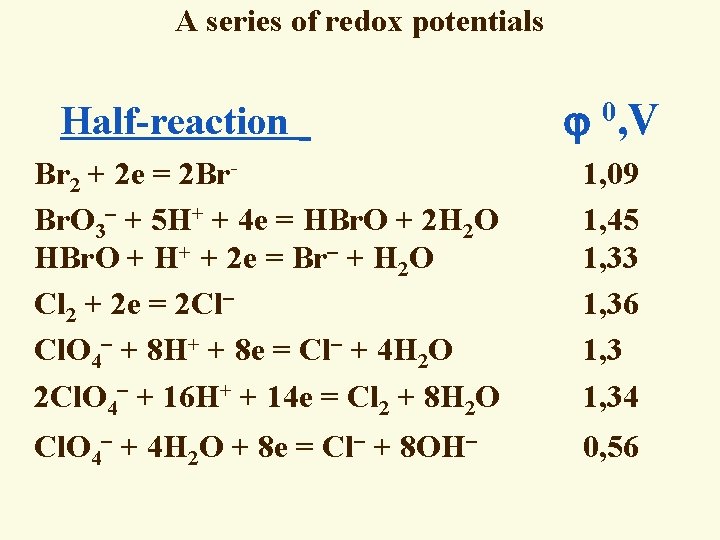
A series of redox potentials Half-reaction 0, V Br 2 + 2 e = 2 Br Br. O 3 + 5 H+ + 4 e = HBr. O + 2 H 2 O HBr. O + H+ + 2 e = Br + H 2 O Cl 2 + 2 e = 2 Cl Cl. O 4 + 8 H+ + 8 e = Cl + 4 H 2 O 2 Cl. O 4 + 16 H+ + 14 e = Cl 2 + 8 H 2 O 1, 09 1, 45 1, 33 1, 36 1, 34 Cl. O 4 + 4 H 2 O + 8 e = Cl + 8 OH 0, 56

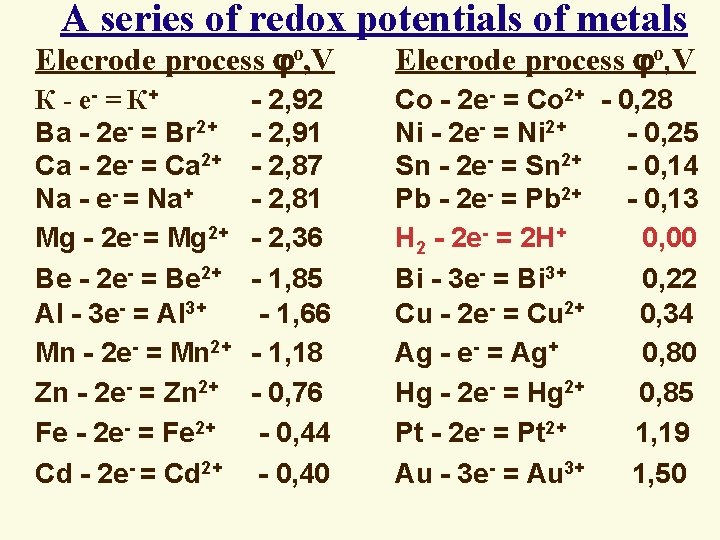
A series of redox potentials of metals Elecrode process о, V К - е- = К+ - 2, 92 Ba - 2 e- = Br 2+ - 2, 91 Ca - 2 e- = Ca 2+ - 2, 87 Na - e- = Na+ - 2, 81 Mg - 2 e- = Mg 2+ - 2, 36 Be - 2 e- = Be 2+ - 1, 85 Al - 3 e- = Al 3+ - 1, 66 Mn - 2 e- = Mn 2+ - 1, 18 Zn - 2 e- = Zn 2+ - 0, 76 Fe - 2 e- = Fe 2+ - 0, 44 Cd - 2 e- = Cd 2+ - 0, 40 Co - 2 e- = Co 2+ - 0, 28 Ni - 2 e- = Ni 2+ - 0, 25 Sn - 2 e- = Sn 2+ - 0, 14 Pb - 2 e- = Pb 2+ - 0, 13 H 2 - 2 e- = 2 H+ 0, 00 Bi - 3 e- = Bi 3+ 0, 22 Cu - 2 e- = Cu 2+ 0, 34 Ag - e- = Ag+ 0, 80 Hg - 2 e- = Hg 2+ 0, 85 Pt - 2 e- = Pt 2+ 1, 19 Au - 3 e- = Au 3+ 1, 50

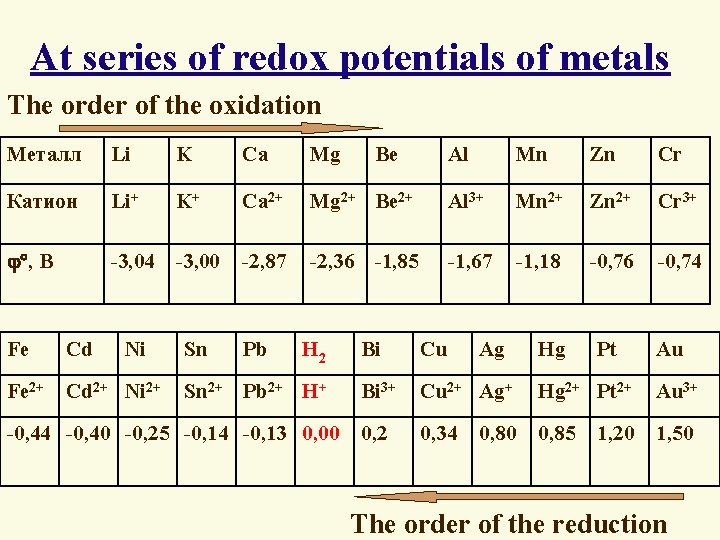
A series of redox potentials of metals Redactive activity metals grow Металл Li K Ca Mg Катион Li+ K+ Ca 2+ , В -3, 04 -3, 00 -2, 87 Ni Sn Fe Cd Fe 2+ Cd 2+ Ni 2+ Pb Al Mn Zn Cr Mg 2+ Be 2+ Al 3+ Mn 2+ Zn 2+ Cr 3+ -2, 36 -1, 85 -1, 67 -1, 18 -0, 76 -0, 74 H 2 Sn 2+ Pb 2+ H+ -0, 44 -0, 40 -0, 25 -0, 14 -0, 13 0, 00 Be Bi Cu Ag Bi 3+ Cu 2+ Ag+ Hg 2+ Pt 2+ Au 3+ 0, 2 0, 34 0, 85 1, 50 0, 80 Oxidative activity cations of metals grow Hg Pt 1, 20 Au

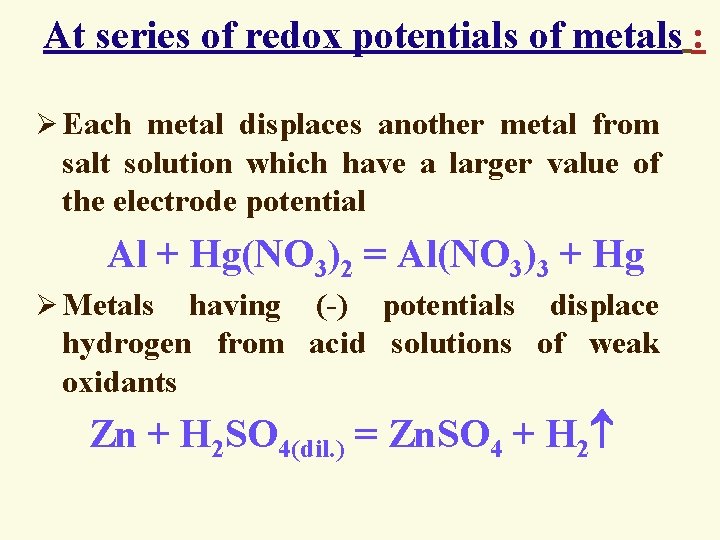
At series of redox potentials of metals : Ø Each metal displaces another metal from salt solution which have a larger value of the electrode potential Al + Hg(NO 3)2 = Al(NO 3)3 + Hg Ø Metals having (-) potentials displace hydrogen from acid solutions of weak oxidants Zn + H 2 SO 4(dil. ) = Zn. SO 4 + H 2

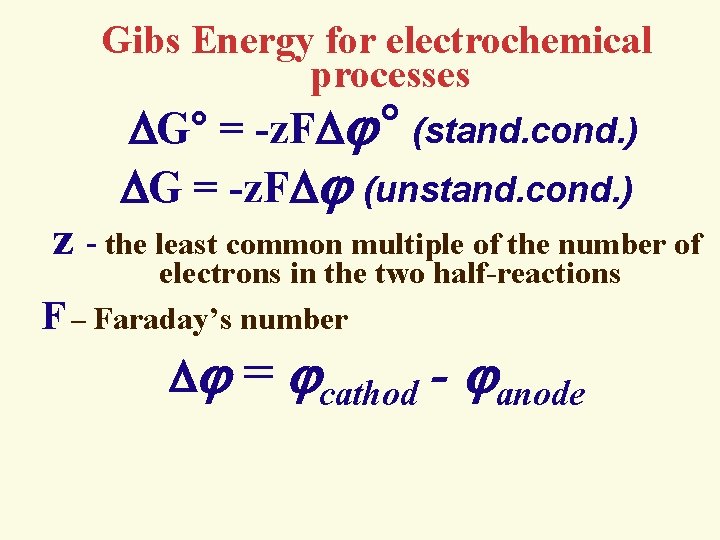
Gibs Energy for electrochemical processes G° = -z. F ° (stand. cond. ) G = -z. F (unstand. cond. ) z - the least common multiple of the number of electrons in the two half-reactions F – Faraday’s number = cathod - anode

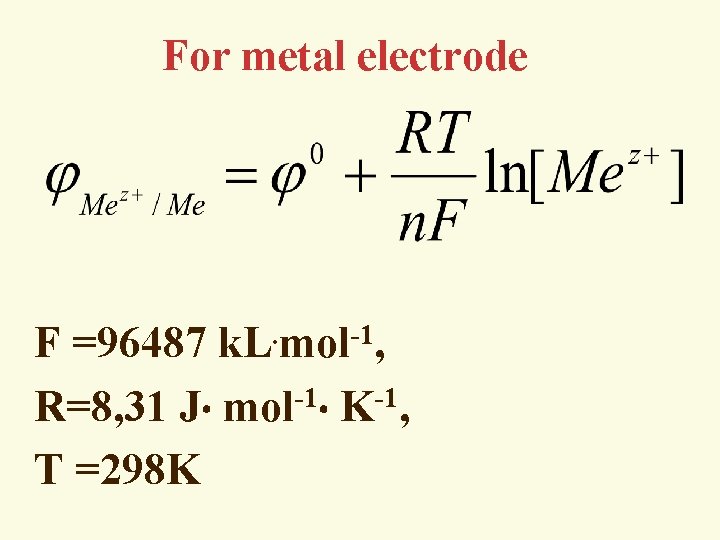
Nernst’s equation is the calculation of an electrode potential of any conditions x RT [Ox] = °+ ln y n. F [Red] [OX] и [Red] equilibrium concentration of the oxidized and reduced forms n- the number of electrons in a half-reaction

For metal electrode . -1 F =96487 k. L mol , -1 -1 R=8, 31 J mol K , T =298 K

Chemical power sources 4 Galvanic Cells 4 Concentration elements 4 Fuel cells 4 Batteries 4 Due to the direct conversion of chemical energy into electrical Chemical power sources have a high coefficient of performance (about 70 -90%)

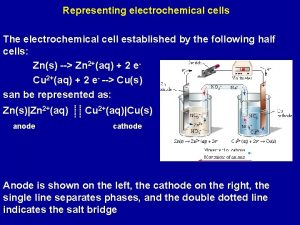
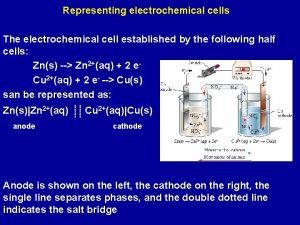
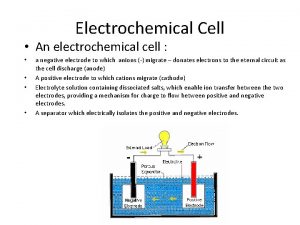
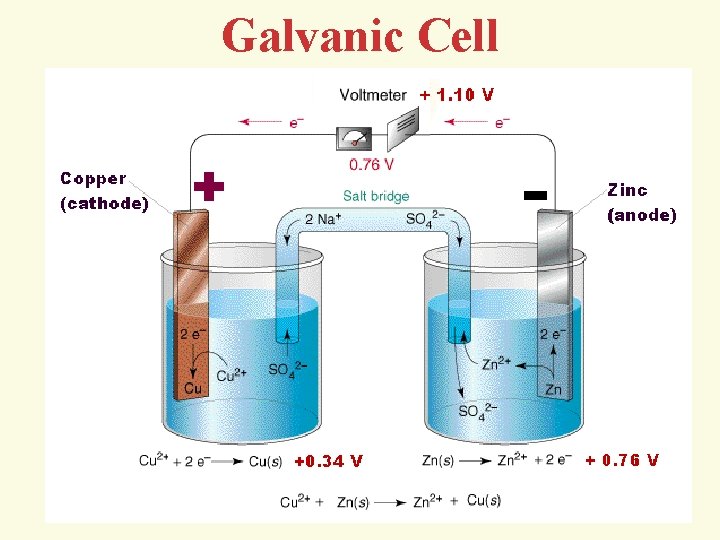
Galvanic Cell

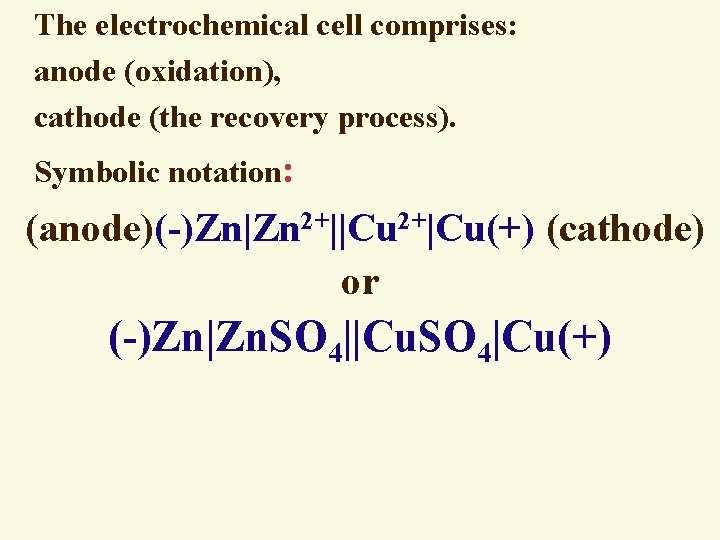
The electrochemical cell comprises: anode (oxidation), cathode (the recovery process). Symbolic notation: (anode)(-)Zn|Zn 2+||Cu 2+|Cu(+) (cathode) or (-)Zn|Zn. SO 4||Cu. SO 4|Cu(+)

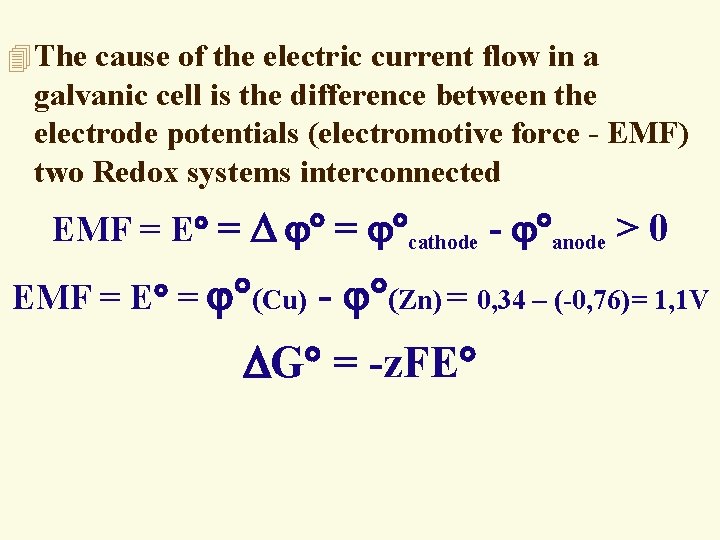
4 The cause of the electric current flow in a galvanic cell is the difference between the electrode potentials (electromotive force - EMF) two Redox systems interconnected EMF = Е = = cathode - аnode > 0 EMF = Е = (Сu) - (Zn) = 0, 34 – (-0, 76)= 1, 1 V G = -z. FЕ

Modern Chemical power sources 4 They have a common electrolyte (alkali) and steel as a construction material buildings and shunts. 4 Material (-) electrode (reducing agent) used Mg, Zn, Pb, Fe, Cd. 4 Material (+) electrode (oxidant) was used Pb. O 2, Hg. O, Mn. O 2, Cu. Cl, etc.

Lead Battery The prosses is based on two reactions: Pb. O 2 + Pb Pb. O Charging Ox. Red Discharging Electrolyte H 2 SO 4 (33 -37%) H 2 SO 4 HSO 4 + H +

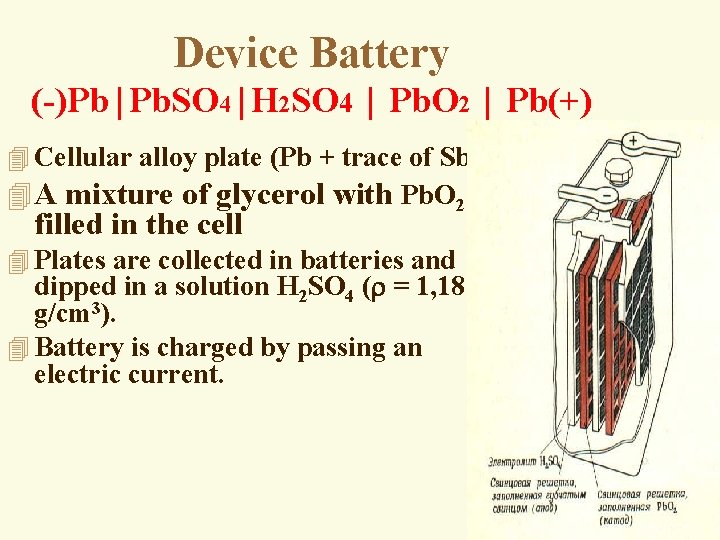
Device Battery (-)Pb│Pb. SO 4│H 2 SO 4 │ Pb. O 2 │ Pb(+) 4 Cellular alloy plate (Pb + trace of Sb). 4 A mixture of glycerol with Pb. O 2 is filled in the cell 4 Plates are collected in batteries and dipped in a solution H 2 SO 4 ( = 1, 18 g/cm 3). 4 Battery is charged by passing an electric current.

Charging Battery 4 (-) cathode: Pb. SO 4 + 2 e = Pb + SO 42 4 (+) anode: Pb. SO 4 - 2 e + 2 H 2 O = Pb. O 2 + SO 42 + 4 H+ Discharging Battery (-) anode: Pb 2 e + SO 42 = Pb. SO 4 (+) cathode: Pb. O 2 + 2 e + SO 42 + 4 H+= Pb. SO 4+ 2 H 2 O For a charged battery: = 2, 1 V When the battery is charging, electrolyte density increases and decreases during discharge. Knowing the density we can judge the state of charge.

Problem n The galvanic cell consists of Mg and Ag electrodes in solutions of their nitrates. The concentration of [Mg 2+] = 0, 5 M; [Ag+] = 2, 0 M. To write the electrochemical scheme of a galvanic cell, the redox reaction and calculate the EMF of a galvanic cell.

Corrosion of Metals

n Corrosion of metals - is the process of spontaneous fracture of metals under the influence of the environment, which is accompanied by the release of energy and material dispersion n Corrosion processes are irreversible, in accordance with the 2 nd law of thermodynamics (entropy increase)

Classification of corrosion processes according to mechanism: 1) chemical a) in solution nonelectrolytes b) gas 2) electrochemical a) atmospheric, b) soil, c) stray currents

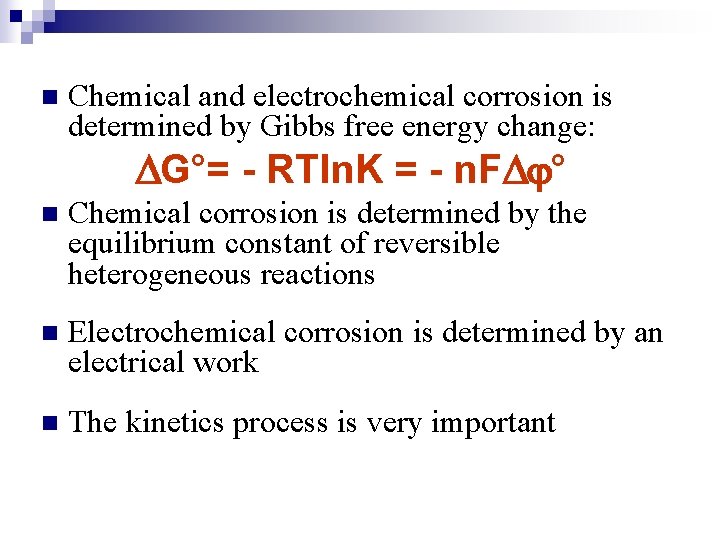
n Chemical and electrochemical corrosion is determined by Gibbs free energy change: G°= - RTln. K = - n. F ° n Chemical corrosion is determined by the equilibrium constant of reversible heterogeneous reactions n Electrochemical corrosion is determined by an electrical work n The kinetics process is very important

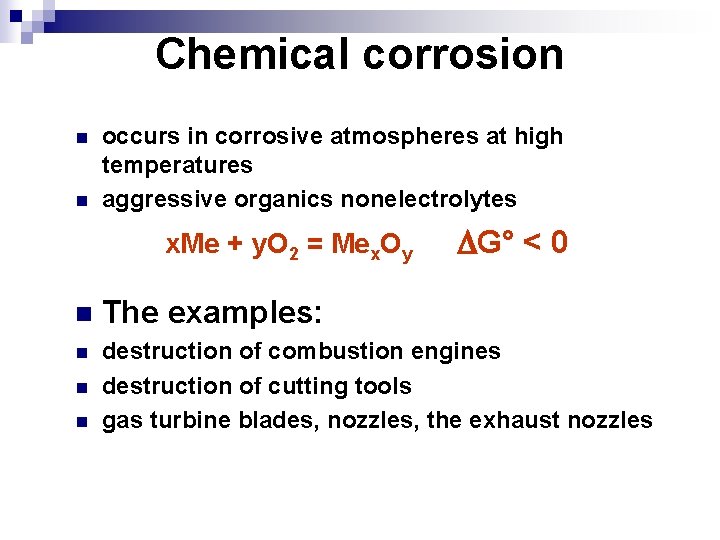
Chemical corrosion n n occurs in corrosive atmospheres at high temperatures aggressive organics nonelectrolytes x. Me + y. O 2 = Mex. Oy G° < 0 n The examples: n destruction of combustion engines destruction of cutting tools gas turbine blades, nozzles, the exhaust nozzles n n

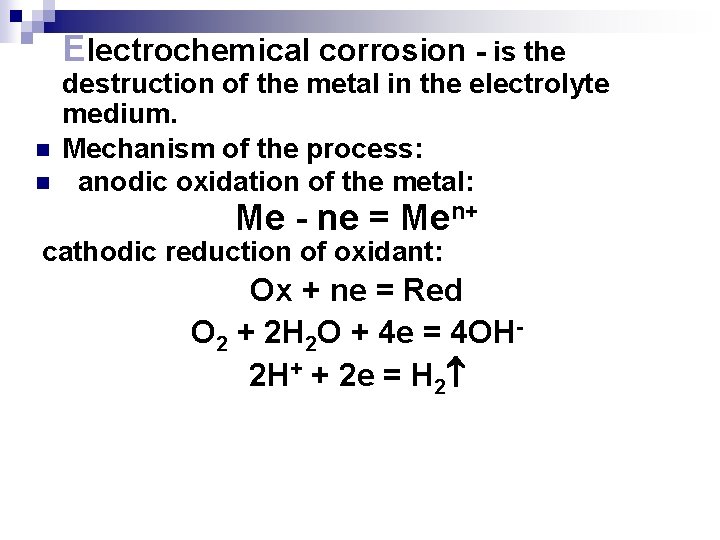
Electrochemical corrosion - is the n n destruction of the metal in the electrolyte medium. Mechanism of the process: anodic oxidation of the metal: Ме - nе = Меn+ cathodic reduction of oxidant: Ox + ne = Red O 2 + 2 H 2 O + 4 e = 4 OH 2 H+ + 2 e = H 2

n n n Processes of electrochemical corrosion are similar to the processes occurring in the cell. There are formed microgalvanopairs. The difference - the absence of the external circuit, i. e. electrons do not come out of the metal but move inside the metal. Chemical energy of oxidation of the metal is not transmitted in work, but only in heat. It is possible the secondary chemical reactions: Men+ + n. OH- = Me(OH)n

Dangerous areas point of contact with different metals n areas with different thermal and mechanical treatment n sections stained with oxides and other mineral pigments n metal alloy inhomogeneity Strong corrosive properties are: n seawater n technological solutions. (acid salts, etc. ) n groundwater n wastewater n damp air n

Corrosion of iron by atmospheric oxygen dissolved in the water air rust Drop of water Ion (Fe)

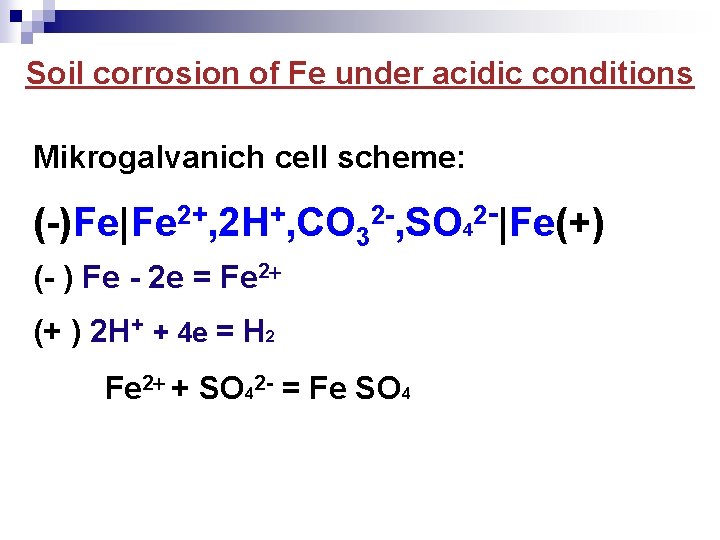
Soil corrosion of Fe under acidic conditions Mikrogalvanich cell scheme: (-)Fe|Fe 2+, 2 H+, СO 32 -, SO 42 -|Fe(+) (- ) Fe - 2 e = Fe 2 (+ ) 2 H+ + 4 e = H 2 Fe 2 + SO 42 - = Fe SO 4

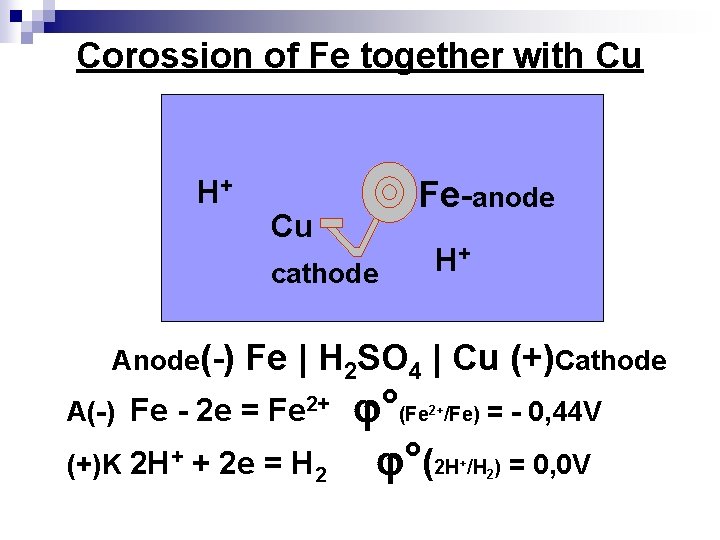
Corossion of Fe together with Cu H+ Cu cathode Fe-anode H+ Anode(-) Fe | H 2 SO 4 | Cu (+)Cathode °(Fe /Fe) = - 0, 44 V (+)K 2 H+ + 2 e = H 2 °(2 H /H ) = 0, 0 V A(-) Fe - 2 e = Fe 2+ 2+ + 2

Methods of Corrosion Protection n n n Creating a rational constructions Impact on the environment Inhibitors Protective Coating: lubrication varnishes, paint, polymers, oxidation, phosphating, metal coatings Protection of the external potential: tread, the current source

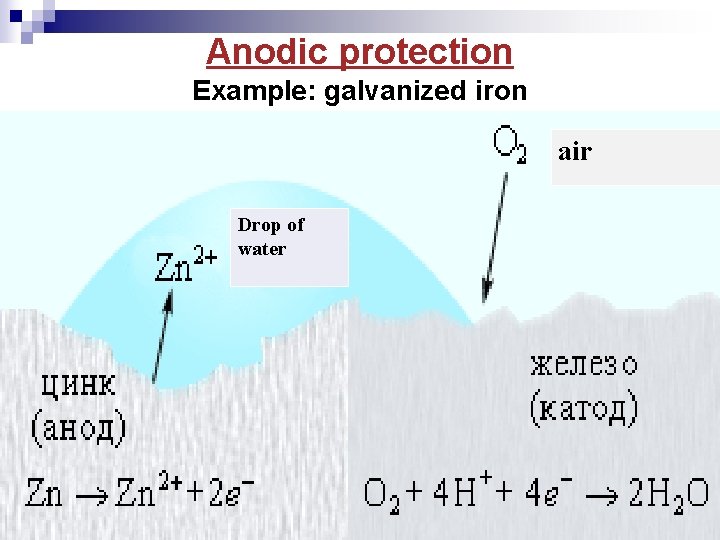
Anodic protection Example: galvanized iron air Drop of water

Cathodic protection Example: Corrosion "tinplate"

Cathodic protection against corrosion

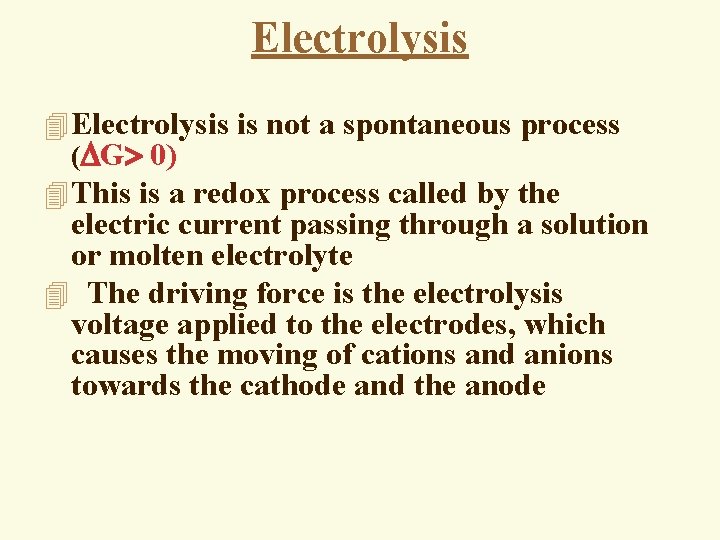
Electrolysis 4 Electrolysis is not a spontaneous process ( G 0) 4 This is a redox process called by the electric current passing through a solution or molten electrolyte 4 The driving force is the electrolysis voltage applied to the electrodes, which causes the moving of cations and anions towards the cathode and the anode

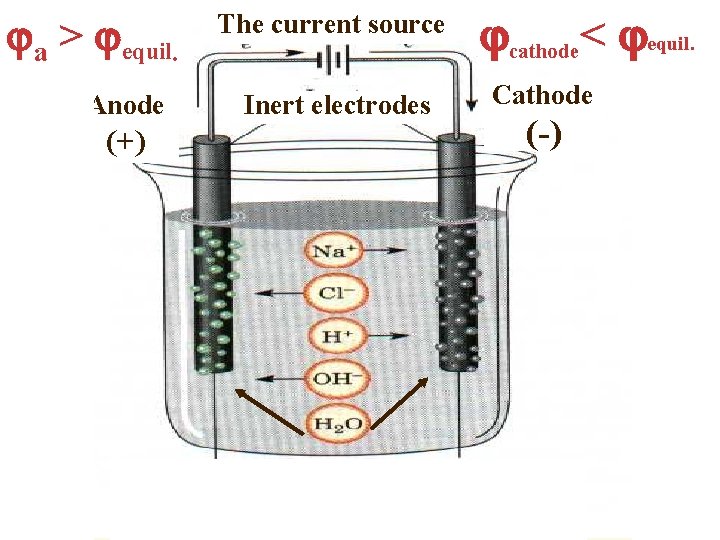
а > equil. Anode (+) The current source Inert electrodes cathode< equil. Cathode (-)

Factors influencing the electrolysis 4 electrolyte composition 4 electrode material 4 temperature 4 voltage 4 the current density etc.

4 Discharging occurs simultaneously with cations discharging anions; therefore externally imposed voltage is divided into two parts extending at an anodic oxidation and cathodic reduction of the ions or molecules sometimes.

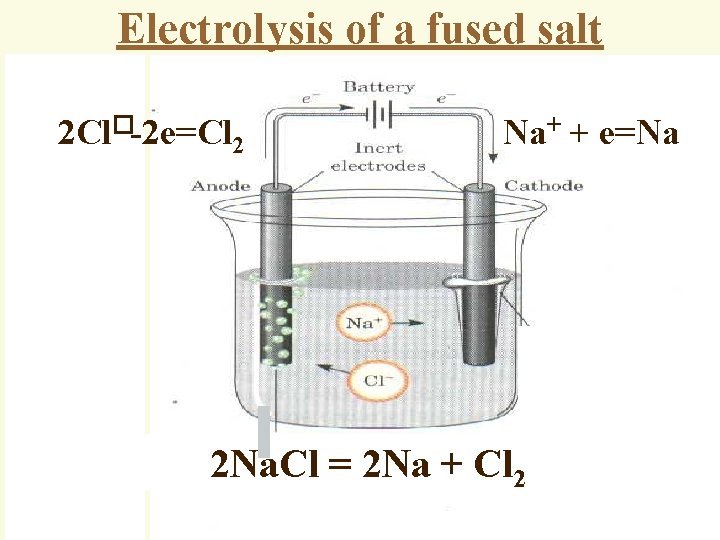
Electrolysis of a fused salt 2 Cl�-2 e=Cl 2 Na+ + e=Na 2 Na. Cl = 2 Na + Cl 2

Sequence of ion discharging If the solution of two or more kinds of cations and anions is most likely the process with a minimum expenditure of energy 4 At the cathode, reducing cation in order to reduce their potential 4 Anions are oxidized at the anode in the ascending order of their potential

At series of redox potentials of metals The order of the oxidation Металл Li K Ca Mg Катион Li+ K+ Ca 2+ , В -3, 04 -3, 00 -2, 87 Ni Sn Fe Cd Fe 2+ Cd 2+ Ni 2+ Pb Al Mn Zn Cr Mg 2+ Be 2+ Al 3+ Mn 2+ Zn 2+ Cr 3+ -2, 36 -1, 85 -1, 67 -1, 18 -0, 76 -0, 74 H 2 Sn 2+ Pb 2+ H+ -0, 44 -0, 40 -0, 25 -0, 14 -0, 13 0, 00 Be Bi Cu Ag Bi 3+ Cu 2+ Ag+ Hg 2+ Pt 2+ Au 3+ 0, 2 0, 34 0, 85 1, 50 0, 80 Hg Pt 1, 20 Au The order of the reduction

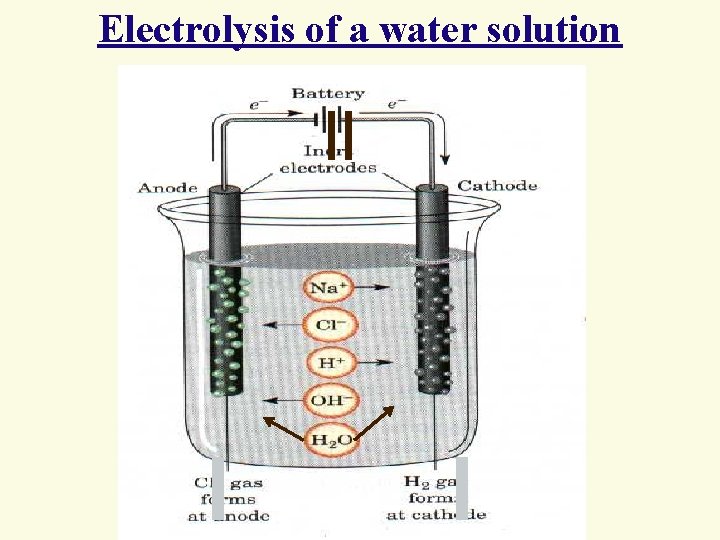
Electrolysis of a water solution

Water, as part of the electrolyte solution in the electrode, participates processes 4 Oxidation of water: 2 H 2 O - 4 e = O 2 + 4 H+ o = + 1, 23 V 4 Portentials: F–, SO 42 -, NO 3 -, PO 43 -, CO 32 - etc. > than + 1, 23 В 4 Consequently, these anions can not be oxidized in aqueous solution

Reduction of water: 2 H 2 O + 2 e = H 2 + 2 OH- 4 in the alkali medium: o = – 0, 828 V 4 In the neutral medium: = – 0, 41 V 4 Potentials of the ions of alkali and alkaline -earth metals, Al, Ti < potential than hydrogen reduction. 4 Consequently, these metals can not be obtained by electrolysis of aqueous solutions of their salts

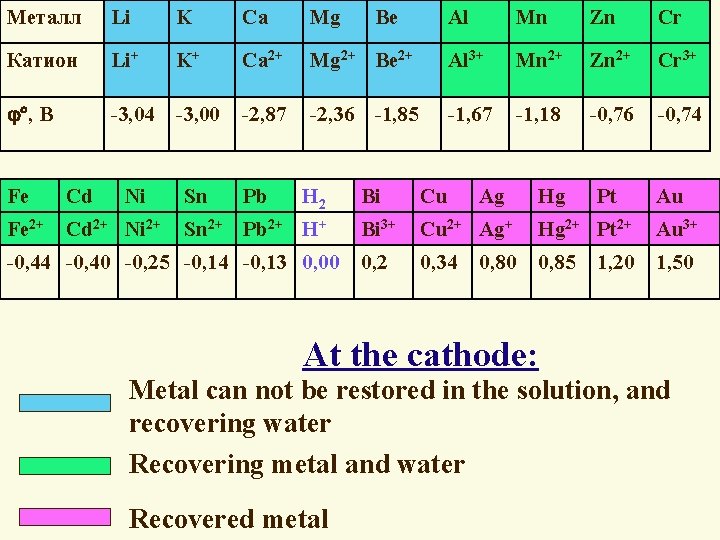
Металл Li K Ca Mg Катион Li+ K+ Ca 2+ , В -3, 04 -3, 00 -2, 87 Ni Sn Fe Cd Fe 2+ Cd 2+ Ni 2+ Pb Al Mn Zn Cr Mg 2+ Be 2+ Al 3+ Mn 2+ Zn 2+ Cr 3+ -2, 36 -1, 85 -1, 67 -1, 18 -0, 76 -0, 74 H 2 Sn 2+ Pb 2+ H+ -0, 44 -0, 40 -0, 25 -0, 14 -0, 13 0, 00 Be Bi Cu Ag Bi 3+ Cu 2+ Ag+ Hg 2+ Pt 2+ Au 3+ 0, 2 0, 34 0, 85 1, 50 0, 80 Hg Pt 1, 20 Au At the cathode: Metal can not be restored in the solution, and recovering water Recovering metal and water Recovered metal

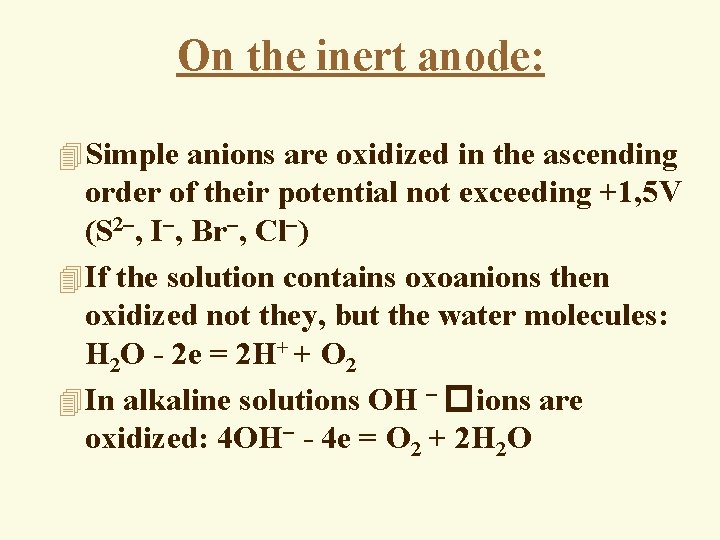
On the inert anode: 4 Simple anions are oxidized in the ascending order of their potential not exceeding +1, 5 V (S 2 , I , Br , Cl ) 4 If the solution contains oxoanions then oxidized not they, but the water molecules: H 2 O - 2 e = 2 H+ + O 2 4 In alkaline solutions OH � ions are oxidized: 4 OH - 4 e = O 2 + 2 H 2 O

Electrolysis with an active anode 4 The anode is made of metal which is capable to oxidize before the particles in the solution cathode: Cu 2+ + 2 e = Cuo anode (Cu): Cuo - 2 e = Cu 2+

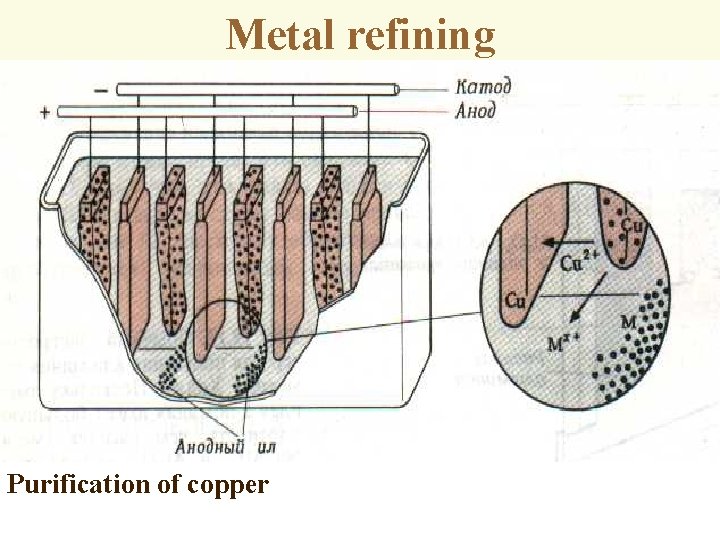
Metal refining Purification of copper
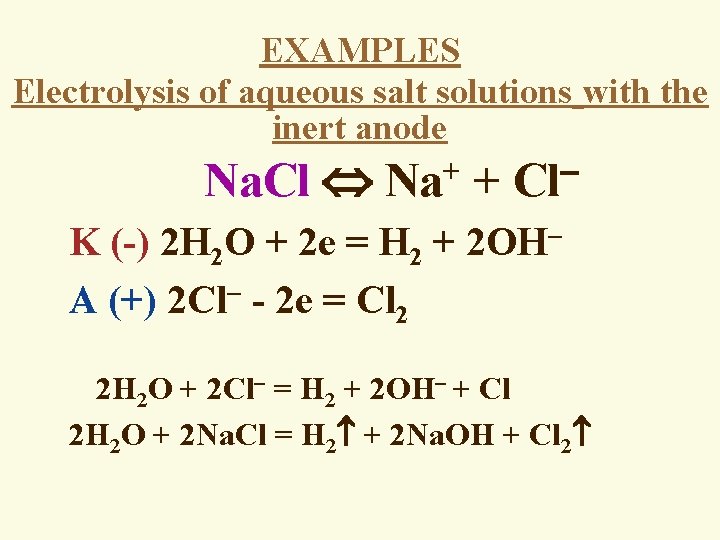
EXAMPLES Electrolysis of aqueous salt solutions with the inert anode Na. Cl Na+ + Cl K (-) 2 H 2 O + 2 e = H 2 + 2 OH A (+) 2 Cl - 2 e = Cl 2 2 H 2 O + 2 Cl = H 2 + 2 OH + Cl 2 H 2 O + 2 Na. Cl = H 2 + 2 Na. OH + Cl 2
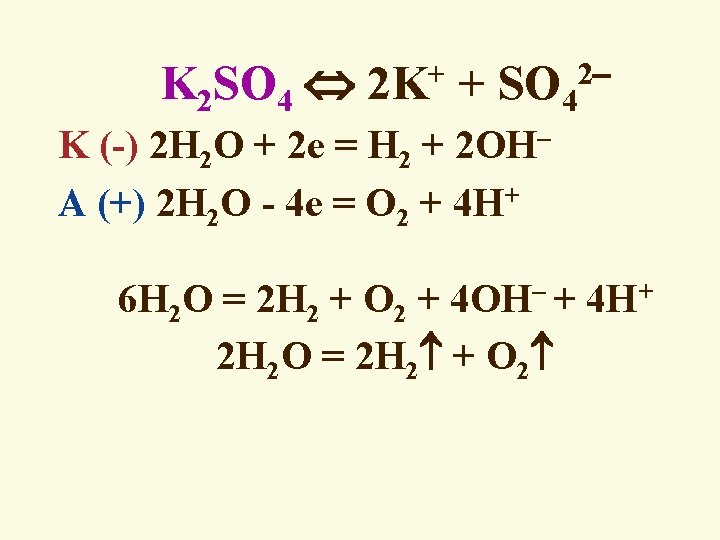
K 2 SO 4 2 K+ + SO 42 K (-) 2 H 2 O + 2 e = H 2 + 2 OH A (+) 2 H 2 O - 4 e = O 2 + 4 H+ 6 H 2 O = 2 H 2 + O 2 + 4 OH + 4 H+ 2 H 2 O = 2 H 2 + O 2
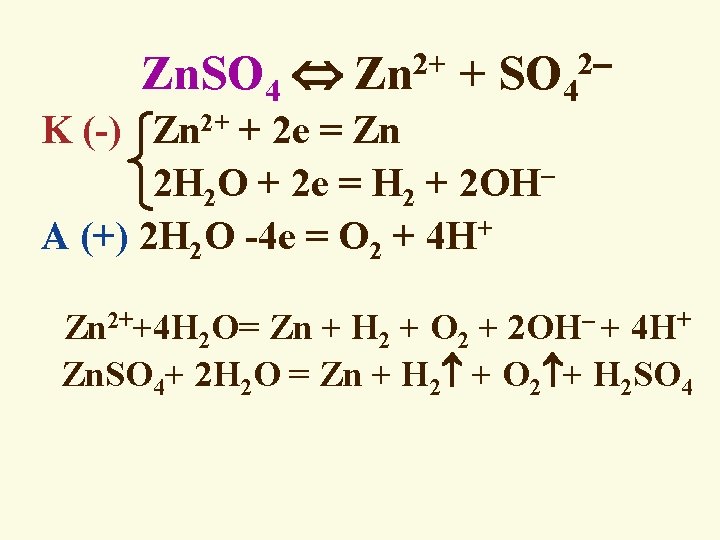
2+ 2 Zn. SO 4 Zn + SO 4 K (-) Zn 2+ + 2 e = Zn 2 H 2 O + 2 e = H 2 + 2 OH A (+) 2 H 2 O -4 e = O 2 + 4 H+ Zn 2++4 H 2 O= Zn + H 2 + O 2 + 2 OH + 4 H+ Zn. SO 4+ 2 H 2 O = Zn + H 2 + O 2 + H 2 SO 4
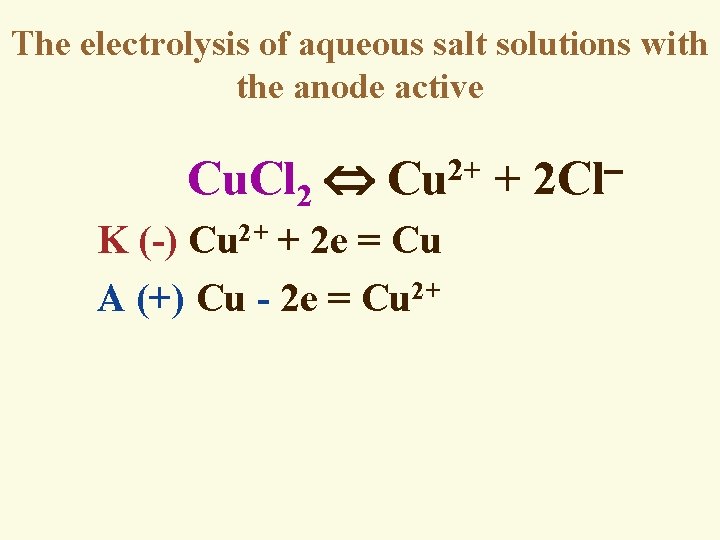
The electrolysis of aqueous salt solutions with the anode active 2+ Сu. Cl 2 Cu + 2 Cl K (-) Cu 2+ + 2 e = Cu A (+) Cu - 2 e = Cu 2+

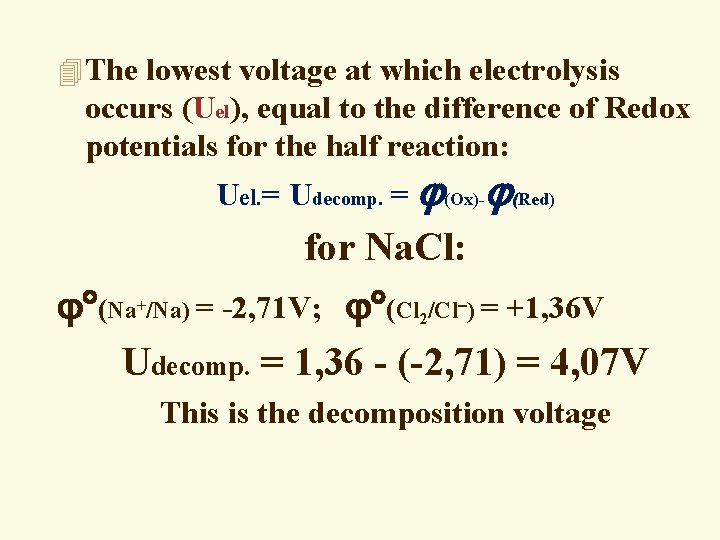
4 The lowest voltage at which electrolysis occurs (Uel), equal to the difference of Redox potentials for the half reaction: Uel. = Udecomp. = (Ox)- (Red) for Na. Cl: (Na /Na) = -2, 71 V; (Cl /Cl ) = +1, 36 V Udecomp. = 1, 36 - (-2, 71) = 4, 07 V + 2 This is the decomposition voltage

Faraday's law (1832 г) 4 Weight converted at the electrode material is proportional to the amount of electricity passing through the solutions or melt 4 When the same amount of electricity passing through different electrolytes formed by an equal number of equivalents of the substance
![Мeq molar mass of the equivalent I electrical current A t Мeq. – molar mass of the equivalent I – electrical current [A] t -](https://slidetodoc.com/presentation_image_h/2f6d474476b681d149e908dbd5c12d5b/image-62.jpg)
Мeq. – molar mass of the equivalent I – electrical current [A] t - time [sеc] F = 96487 k. L - Faraday’s constant – this quantity of electricity which is necessary for the preparation of 1 equivalent of a substance

Problem 1. 4 In what sequence will be reduced the metal ions: K+, Cu 2+, Ag+, Zn 2+, if they are 4 a) melt 4 b) in solution 4 by passing electric current through them.

Problem 2. 4 Describe the electrolysis of aqueous solutions: а) Ni. Cl 2 б) Ni(NO 3)2 в) Li. Cl г) Na 3 PO 4 д) Na. OH (расплав)

Problem 3. 4 Current of 6 A runs for 1. 5 hours in a dilute solution of H 2 SO 4. Calculate the mass of decomposed water and volume H 2 and O 2 evolved at the electrodes.
 Mikael ferm
Mikael ferm Concurrent in os
Concurrent in os Electrochemical impulse
Electrochemical impulse Electrochemical series order
Electrochemical series order Balance redox
Balance redox Electrochemical series
Electrochemical series Electrochemical machining advantages and disadvantages
Electrochemical machining advantages and disadvantages Prevention of corrosion
Prevention of corrosion Concentration of cells
Concentration of cells Waterline corrosion
Waterline corrosion Impedance definition
Impedance definition What is electrochemistry?
What is electrochemistry? Types of electrochemical sensors
Types of electrochemical sensors The stage that creates an “electrochemical gradient”. *
The stage that creates an “electrochemical gradient”. * Electrochemical etch stop
Electrochemical etch stop Electrochemical gradient
Electrochemical gradient Electrochemical machining animation
Electrochemical machining animation Differences between nervous system and endocrine
Differences between nervous system and endocrine The electrochemical series table
The electrochemical series table Electrochemical impedance spectroscopy
Electrochemical impedance spectroscopy Cathode vs anode equation
Cathode vs anode equation How breathalyzer electrochemical
How breathalyzer electrochemical What are electrochemical series
What are electrochemical series Giner electrochemical systems
Giner electrochemical systems Refractory period anatomy
Refractory period anatomy Types of electrochemical corrosion
Types of electrochemical corrosion Diffusion limited
Diffusion limited Electrochemical impulse
Electrochemical impulse Electrochemical deposition
Electrochemical deposition Electrochemical gradient
Electrochemical gradient Ví dụ về giọng cùng tên
Ví dụ về giọng cùng tên Thể thơ truyền thống
Thể thơ truyền thống 101012 bằng
101012 bằng Chúa yêu trần thế
Chúa yêu trần thế Hươu thường đẻ mỗi lứa mấy con
Hươu thường đẻ mỗi lứa mấy con Diễn thế sinh thái là
Diễn thế sinh thái là đại từ thay thế
đại từ thay thế Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Công thức tính thế năng
Công thức tính thế năng Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Lời thề hippocrates
Lời thề hippocrates Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Phản ứng thế ankan
Phản ứng thế ankan Các môn thể thao bắt đầu bằng từ đua
Các môn thể thao bắt đầu bằng từ đua Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Sự nuôi và dạy con của hổ
Sự nuôi và dạy con của hổ Dạng đột biến một nhiễm là
Dạng đột biến một nhiễm là điện thế nghỉ
điện thế nghỉ Nguyên nhân của sự mỏi cơ sinh 8
Nguyên nhân của sự mỏi cơ sinh 8 Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Gấu đi như thế nào
Gấu đi như thế nào Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Một số thể thơ truyền thống
Một số thể thơ truyền thống Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Frameset trong html5
Frameset trong html5 Hệ hô hấp
Hệ hô hấp Tư thế ngồi viết
Tư thế ngồi viết Số nguyên là gì
Số nguyên là gì đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 Tư thế worms-breton
Tư thế worms-breton ưu thế lai là gì
ưu thế lai là gì