Electrochemical Impedance Spectroscopy and Electrochemical Noise Analysis Michele








- Slides: 56

Electrochemical Impedance Spectroscopy and Electrochemical Noise Analysis Michele Curioni Corrosion and Protection Centre University of Manchester UK DICMAPI Seminars 3 rd-4 th December 2014, Naples

Overview (2 days) • Background: DC techniques – Linear polarization / potentiodynamic polarization • Electrochemical Impedance – Concept – Equivalent circuits – Measurement • Electrochemical Noise – Generation of noise – Measurement and analysis DICMAPI Seminars 3 rd-4 th December 2014, Naples

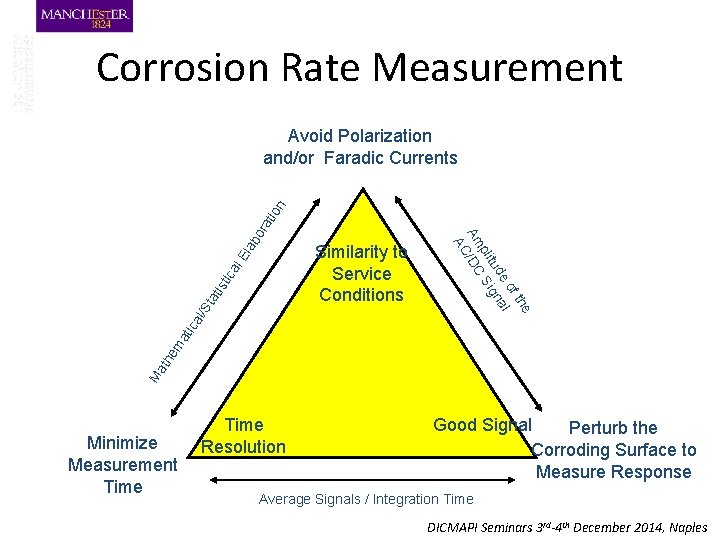
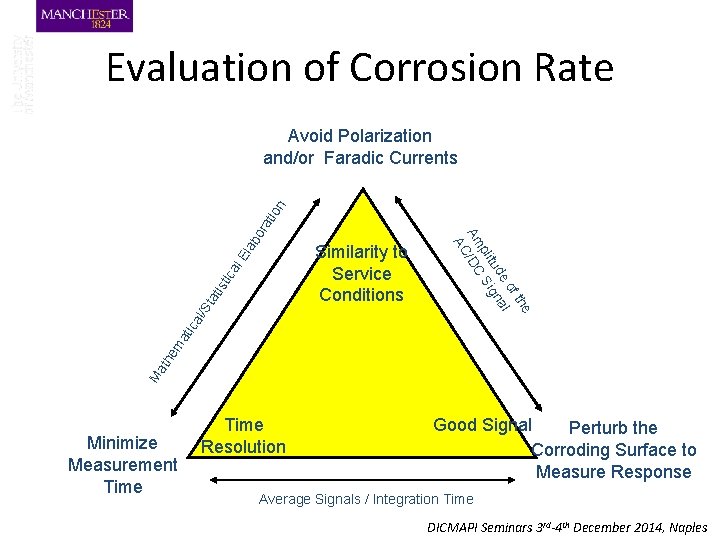
Corrosion Rate Measurement l. E ca sti ati St al/ Ma the ma tic Similarity to Service Conditions e f th e o al tud gn pli Si Am /DC AC lab or ati on Avoid Polarization and/or Faradic Currents Minimize Measurement Time Resolution Good Signal Perturb the Corroding Surface to Measure Response Average Signals / Integration Time DICMAPI Seminars 3 rd-4 th December 2014, Naples

Overview • • Anodic and Cathodic Reactions DC techniques EIS Basic Equivalent Circuit elements DICMAPI Seminars 3 rd-4 th December 2014, Naples

Electrochemical Reaction 2 e- Cu 0 Cu 2+ Anodic Reaction 2 e- Cu 0 Cu 2+ Cu 0 2 e- Cu 0 Cu 2+ 2 e- Cu 0 Cu 2++ 2 e- Cathodic Reaction Cu 2++ 2 e- Cu 0 DICMAPI Seminars 3 rd-4 th December 2014, Naples

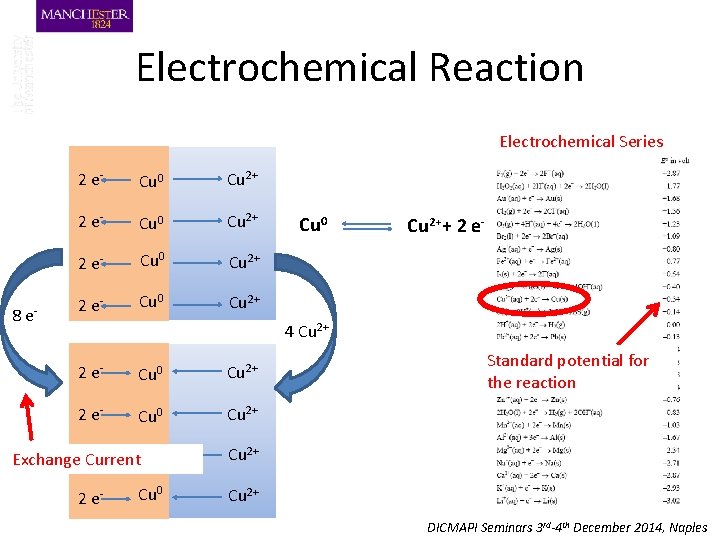
Electrochemical Reaction Electrochemical Series 8 e- 2 e- Cu 0 Cu 2+ Cu 0 Cu 2++ 2 e- 4 Cu 2+ 2 e- Cu 0 Cu 2+ Cu 0 2 e. Exchange Current Cu 2+ Cu 0 Cu 2+ 2 e- Standard potential for the reaction DICMAPI Seminars 3 rd-4 th December 2014, Naples

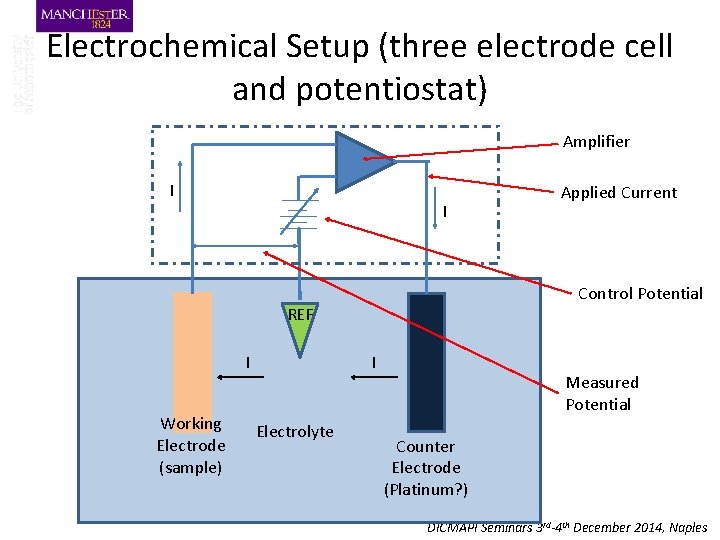
Electrochemical Setup (three electrode cell and potentiostat) Amplifier I I Control Potential REF I Working Electrode (sample) I Electrolyte Applied Current Measured Potential Counter Electrode (Platinum? ) DICMAPI Seminars 3 rd-4 th December 2014, Naples

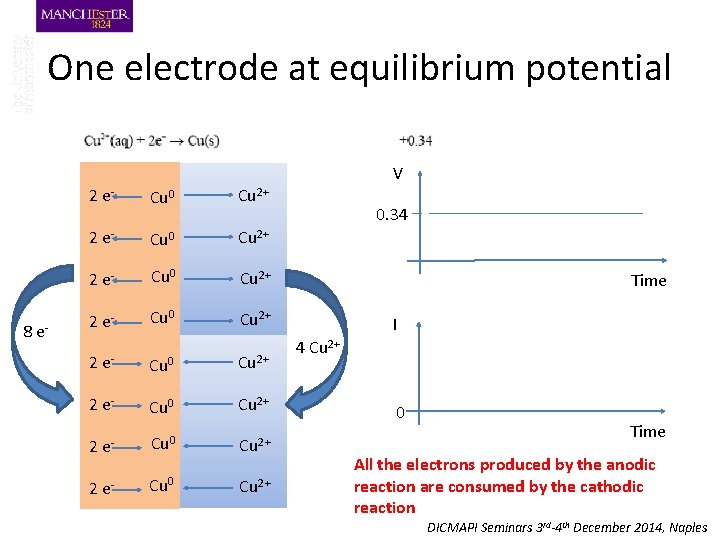
One electrode at equilibrium potential V 8 e- 2 e- Cu 0 Cu 2+ 2 e- Cu 0 Cu 2+ 0. 34 Time I 4 Cu 2+ 0 Time All the electrons produced by the anodic reaction are consumed by the cathodic reaction DICMAPI Seminars 3 rd-4 th December 2014, Naples

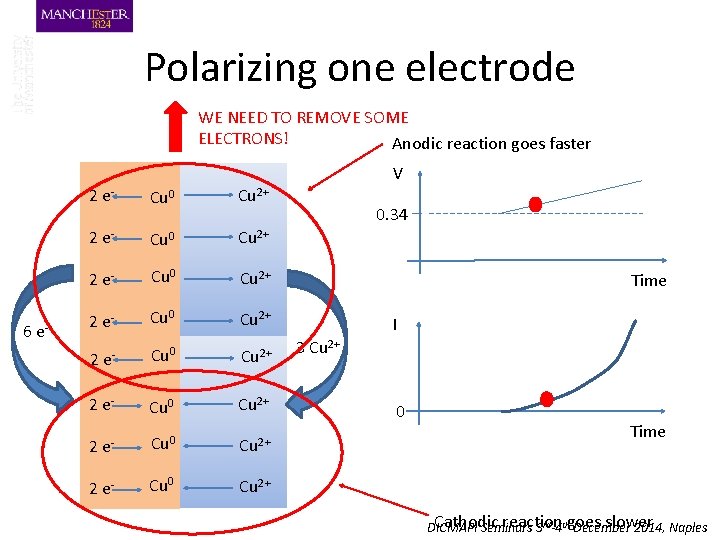
Polarizing one electrode WE NEED TO REMOVE SOME ELECTRONS! Anodic reaction goes faster V 6 e- 2 e- Cu 0 Cu 2+ 2 e- Cu 0 Cu 2+ 0. 34 Time I 3 Cu 2+ 0 Time Cathodic reaction goes slower DICMAPI Seminars 3 rd-4 th December 2014, Naples

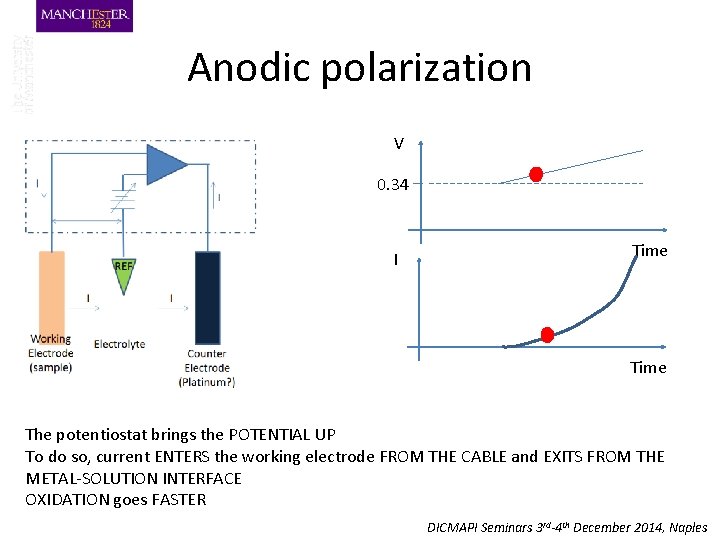
Anodic polarization V 0. 34 I Time The potentiostat brings the POTENTIAL UP To do so, current ENTERS the working electrode FROM THE CABLE and EXITS FROM THE METAL-SOLUTION INTERFACE OXIDATION goes FASTER DICMAPI Seminars 3 rd-4 th December 2014, Naples

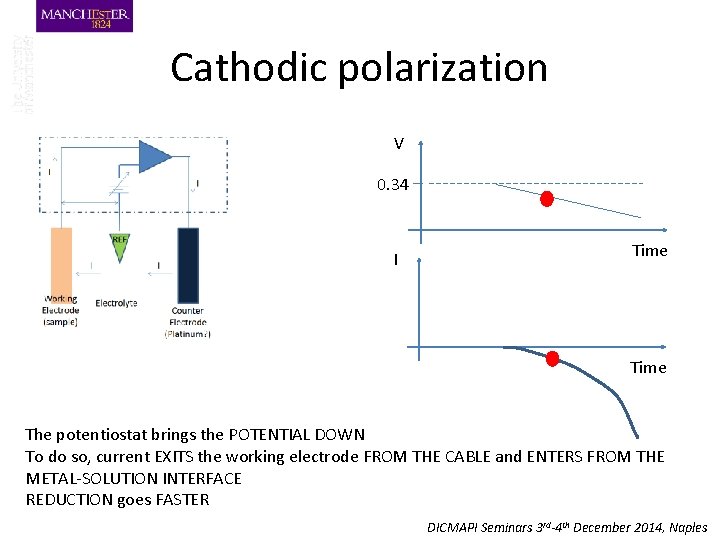
Cathodic polarization V 0. 34 I Time The potentiostat brings the POTENTIAL DOWN To do so, current EXITS the working electrode FROM THE CABLE and ENTERS FROM THE METAL-SOLUTION INTERFACE REDUCTION goes FASTER DICMAPI Seminars 3 rd-4 th December 2014, Naples

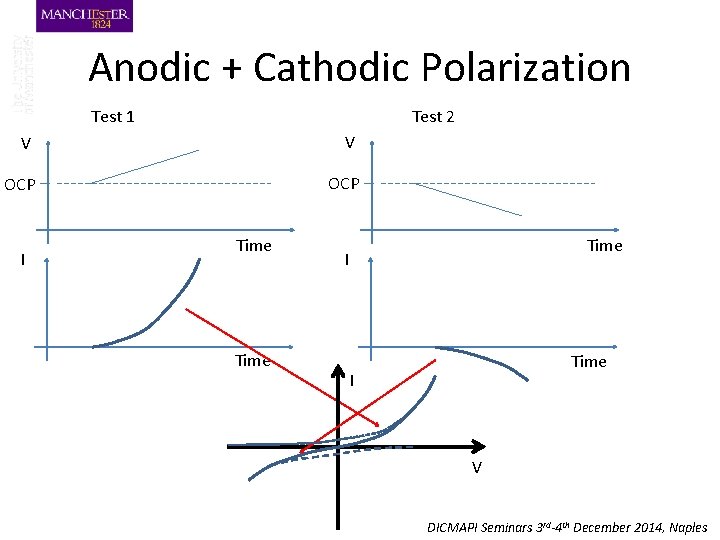
Anodic + Cathodic Polarization Test 1 Test 2 V V OCP I Time I V DICMAPI Seminars 3 rd-4 th December 2014, Naples

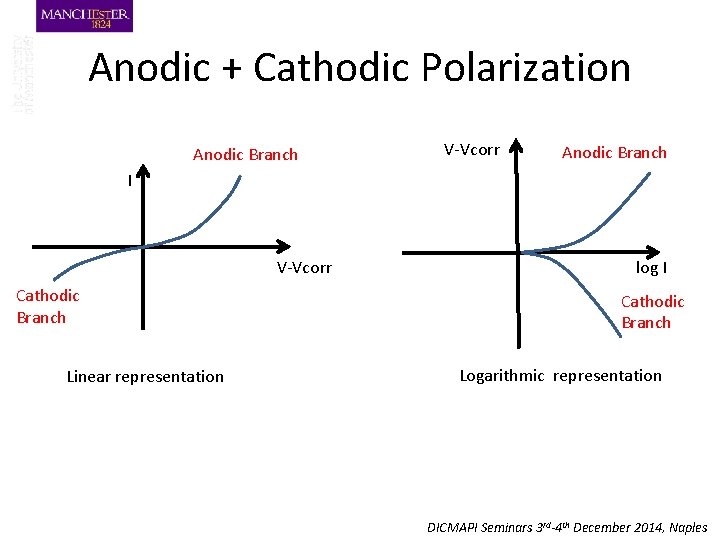
Anodic + Cathodic Polarization Anodic Branch V-Vcorr Anodic Branch I V-Vcorr Cathodic Branch Linear representation log I Cathodic Branch Logarithmic representation DICMAPI Seminars 3 rd-4 th December 2014, Naples

What happens during corrosion V 4 OH- Cathodic Reaction O 2(g) Oxygen Evolution Oxygen Reduction log I 2 H 2 O(l) e- Me Metal Oxidation Men+ Anodic Reaction Metal Reduction DICMAPI Seminars 3 rd-4 th December 2014, Naples

What happens during corrosion V 4 OH- Cathodic Reaction O 2(g) Oxygen Reduction log I 2 H 2 O(l) Corrosion Potential e- Me Metal Oxidation Men+ Corrosion Current Anodic Reaction We can physically measure ONLY THE RED LINE!!! DICMAPI Seminars 3 rd-4 th December 2014, Naples

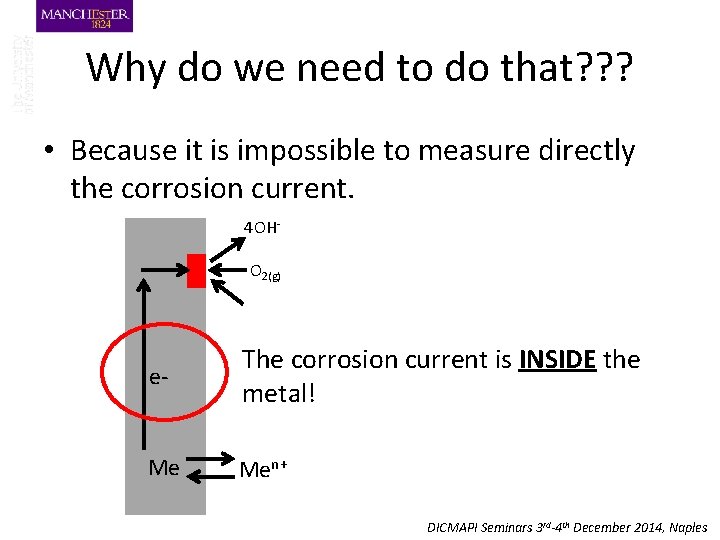
Why do we need to do that? ? ? • Because it is impossible to measure directly the corrosion current. 4 OHO 2(g) e- The corrosion current is INSIDE the metal! Me Men+ DICMAPI Seminars 3 rd-4 th December 2014, Naples

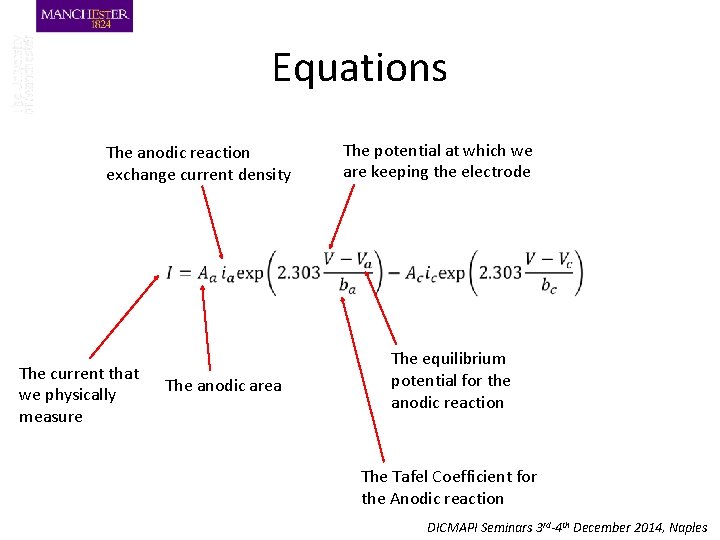
Equations The anodic reaction exchange current density The potential at which we are keeping the electrode The current that we physically measure The anodic area The equilibrium potential for the anodic reaction The Tafel Coefficient for the Anodic reaction DICMAPI Seminars 3 rd-4 th December 2014, Naples

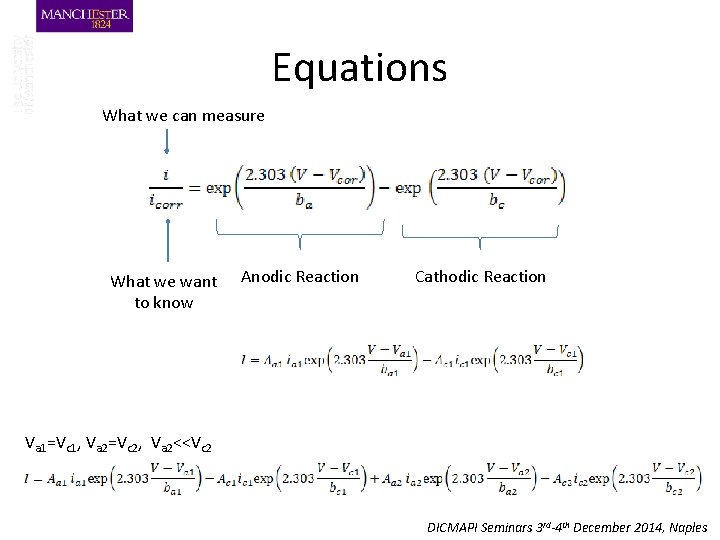
Equations What we can measure What we want to know Anodic Reaction Cathodic Reaction Va 1=Vc 1, Va 2=Vc 2, Va 2<<Vc 2 DICMAPI Seminars 3 rd-4 th December 2014, Naples

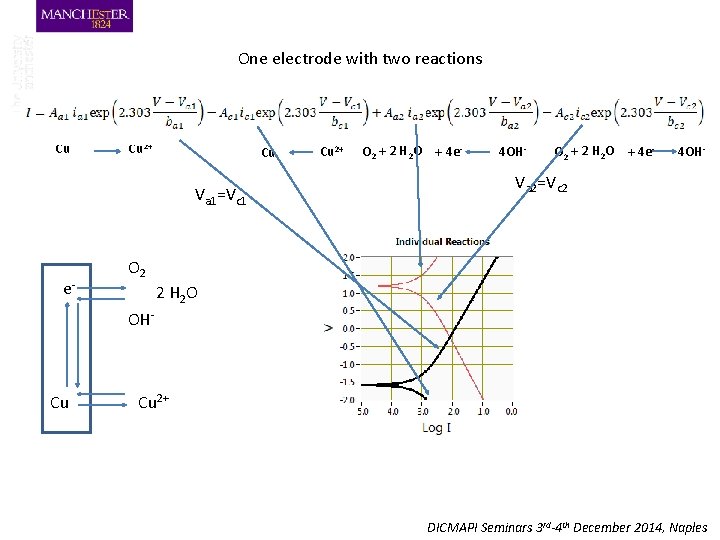
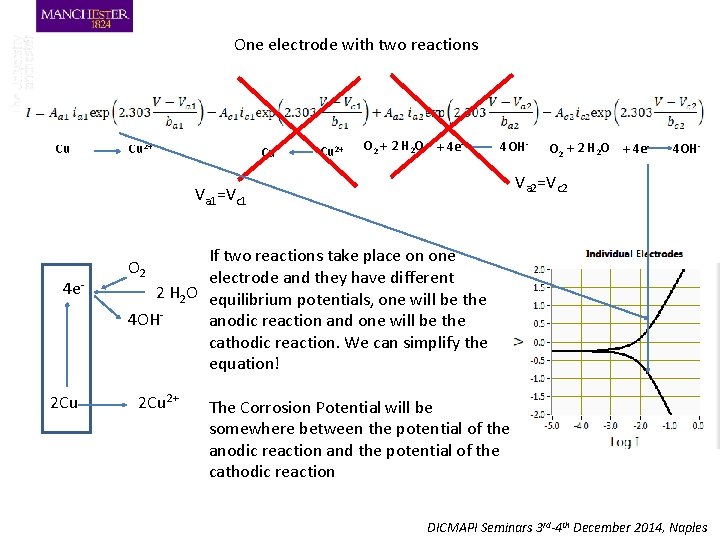
One electrode with two reactions Cu Cu 2+ Cu Va 1=Vc 1 e- Cu 2+ O 2 + 2 H 2 O + 4 e- 4 OH- Va 2=Vc 2 O 2 2 H 2 O OH- Cu Cu 2+ DICMAPI Seminars 3 rd-4 th December 2014, Naples

One electrode with two reactions Cu Cu 2+ Cu Va 1=Vc 1 4 e- 2 Cu Cu 2+ O 2 + 2 H 2 O + 4 e- 4 OH- Va 2=Vc 2 O 2 2 H 2 O 4 OH- 2 Cu 2+ DICMAPI Seminars 3 rd-4 th December 2014, Naples

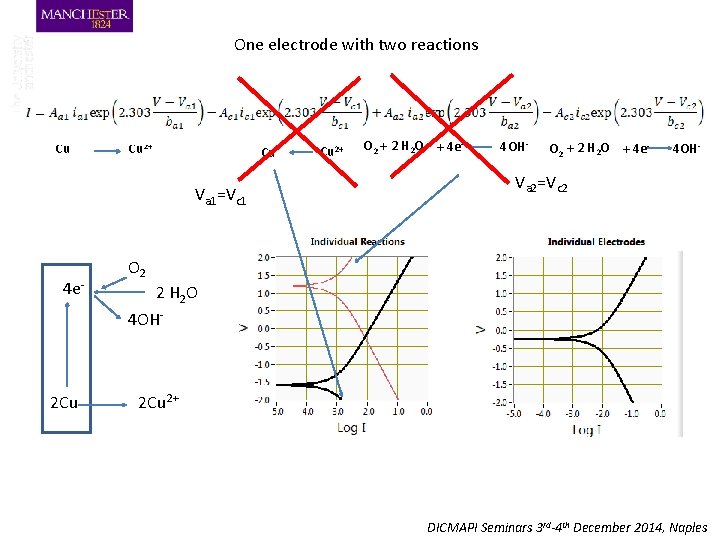
One electrode with two reactions Cu Cu 2+ O 2 + 2 H 2 O + 4 e- 4 OH- 2 Cu 4 OH- Va 2=Vc 2 Va 1=Vc 1 4 e- O 2 + 2 H 2 O + 4 e- If two reactions take place on one electrode and they have different 2 H 2 O equilibrium potentials, one will be the 4 OHanodic reaction and one will be the cathodic reaction. We can simplify the equation! O 2 2 Cu 2+ The Corrosion Potential will be somewhere between the potential of the anodic reaction and the potential of the cathodic reaction DICMAPI Seminars 3 rd-4 th December 2014, Naples

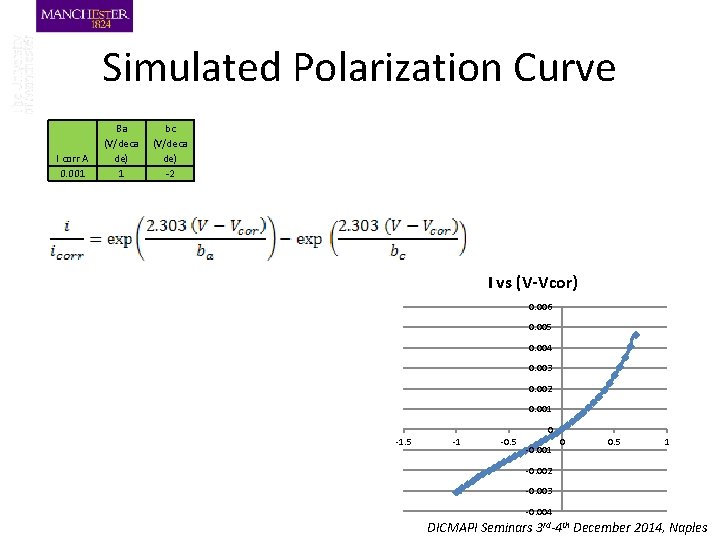
Simulated Polarization Curve I corr A 0. 001 Ba (V/deca de) 1 bc (V/deca de) -2 I vs (V-Vcor) 0. 006 0. 005 0. 004 0. 003 0. 002 0. 001 0 -1. 5 -1 -0. 5 -0. 001 0 0. 5 1 -0. 002 -0. 003 -0. 004 DICMAPI Seminars 3 rd-4 th December 2014, Naples

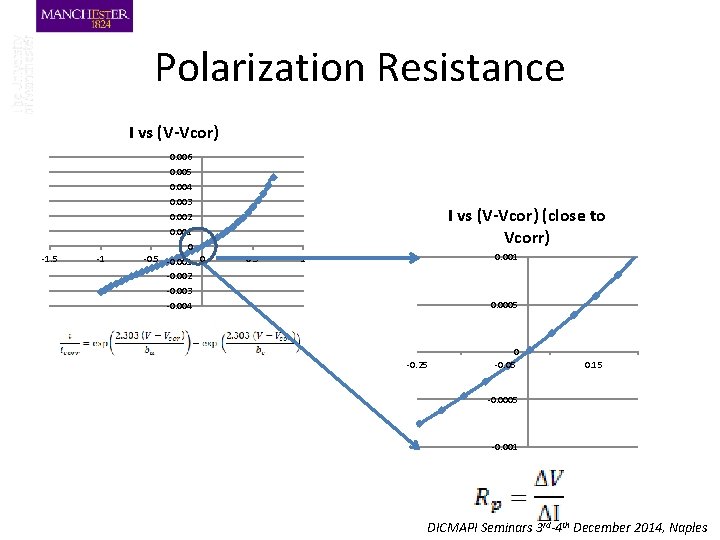
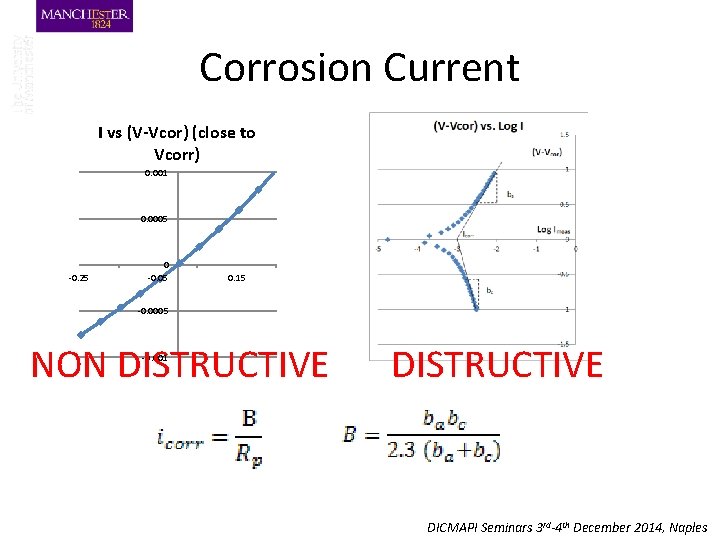
Polarization Resistance I vs (V-Vcor) -1. 5 -1 0. 006 0. 005 0. 004 0. 003 0. 002 0. 001 0 -0. 5 -0. 001 0 -0. 002 -0. 003 -0. 004 I vs (V-Vcor) (close to Vcorr) 0. 5 0. 001 1 0. 0005 -0. 25 0 -0. 05 0. 15 -0. 0005 -0. 001 DICMAPI Seminars 3 rd-4 th December 2014, Naples

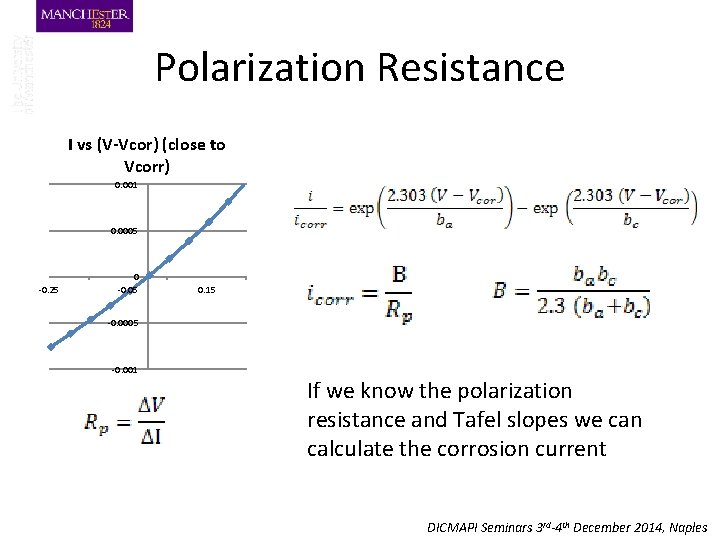
Polarization Resistance I vs (V-Vcor) (close to Vcorr) 0. 001 0. 0005 -0. 25 0 -0. 05 0. 15 -0. 0005 -0. 001 If we know the polarization resistance and Tafel slopes we can calculate the corrosion current DICMAPI Seminars 3 rd-4 th December 2014, Naples

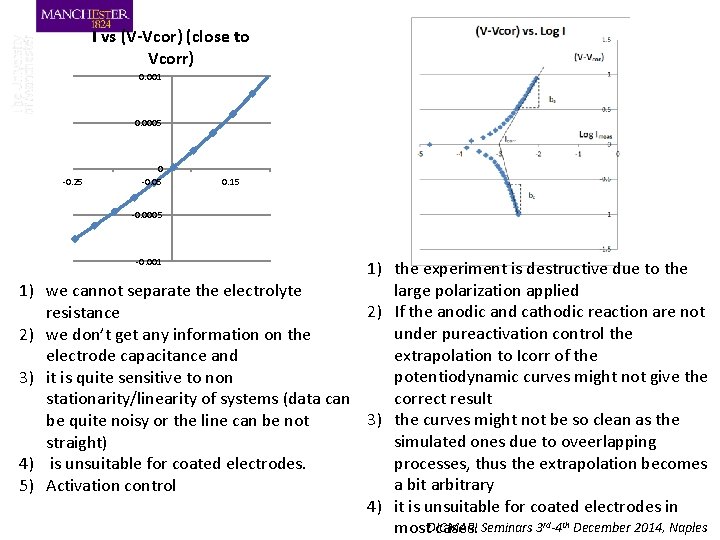
Corrosion Current I vs (V-Vcor) (close to Vcorr) 0. 001 0. 0005 -0. 25 0 -0. 05 0. 15 -0. 0005 NON DISTRUCTIVE -0. 001 DISTRUCTIVE DICMAPI Seminars 3 rd-4 th December 2014, Naples

I vs (V-Vcor) (close to Vcorr) 0. 001 0. 0005 -0. 25 0 -0. 05 0. 15 -0. 0005 -0. 001 1) the experiment is destructive due to the large polarization applied 1) we cannot separate the electrolyte 2) If the anodic and cathodic reaction are not resistance under pureactivation control the 2) we don’t get any information on the extrapolation to Icorr of the electrode capacitance and potentiodynamic curves might not give the 3) it is quite sensitive to non correct result stationarity/linearity of systems (data can 3) the curves might not be so clean as the be quite noisy or the line can be not simulated ones due to oveerlapping straight) processes, thus the extrapolation becomes 4) is unsuitable for coated electrodes. a bit arbitrary 5) Activation control 4) it is unsuitable for coated electrodes in DICMAPI Seminars 3 rd-4 th December 2014, Naples most cases.

Summary DC techniques • The purpose of most electrochemical techniques is to obtain a value of corrosion current. • If you have a value of polarization resistance and have the Tafel coefficients you can calculate the corrosion current. • Potentiodynamic polarization is destructive due to large current applied • Linear polarization is less destructive but might suffer from non stationarity DICMAPI Seminars 3 rd-4 th December 2014, Naples

Evaluation of Corrosion Rate l. E ca sti ati St al/ Ma the ma tic Similarity to Service Conditions e f th e o al tud gn pli Si Am /DC AC lab or ati on Avoid Polarization and/or Faradic Currents Minimize Measurement Time Resolution Good Signal Perturb the Corroding Surface to Measure Response Average Signals / Integration Time DICMAPI Seminars 3 rd-4 th December 2014, Naples

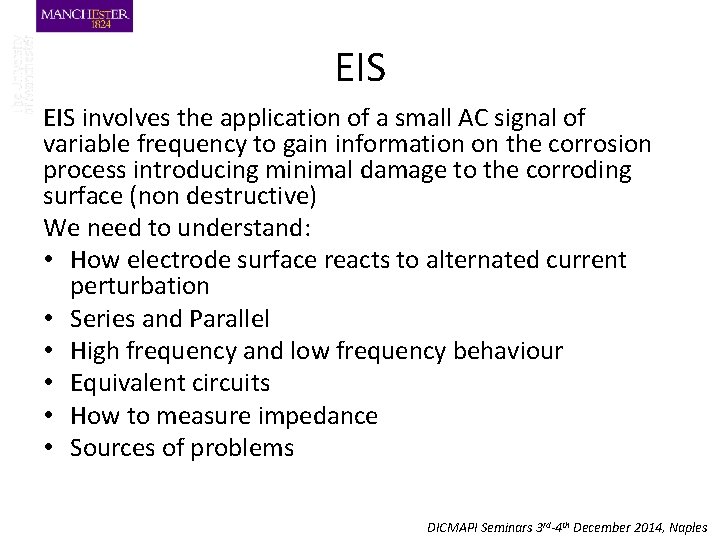
EIS involves the application of a small AC signal of variable frequency to gain information on the corrosion process introducing minimal damage to the corroding surface (non destructive) We need to understand: • How electrode surface reacts to alternated current perturbation • Series and Parallel • High frequency and low frequency behaviour • Equivalent circuits • How to measure impedance • Sources of problems DICMAPI Seminars 3 rd-4 th December 2014, Naples

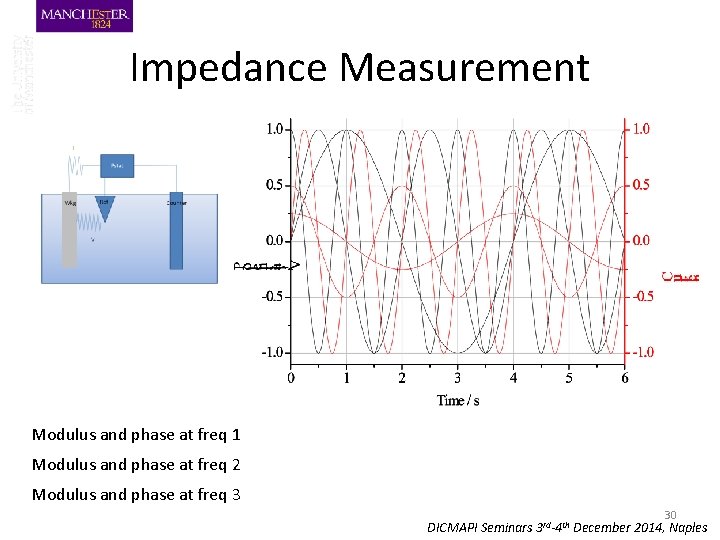
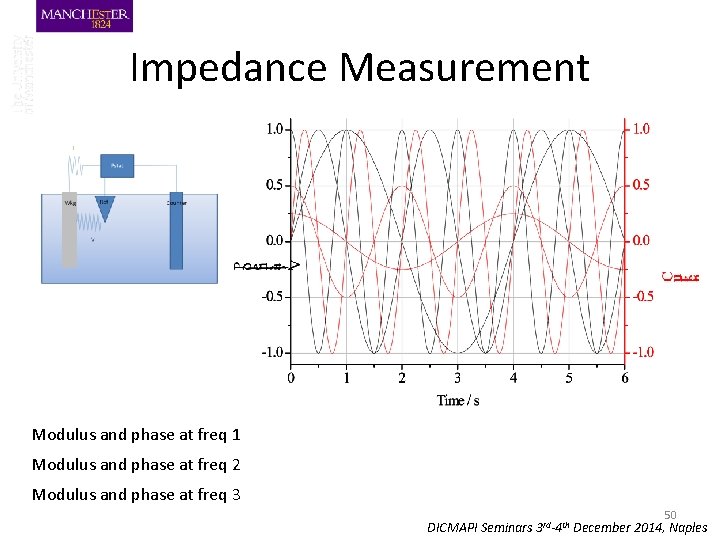
Impedance Measurement Modulus and phase at freq 1 Modulus and phase at freq 2 Modulus and phase at freq 3 30 DICMAPI Seminars 3 rd-4 th December 2014, Naples

Alternated Current Perturbations I vs (V-Vcor) (close to Vcorr) 0. 001 0. 0005 -0. 25 0 -0. 05 0. 15 -0. 0005 -0. 001 Low Frequency DICMAPI Seminars 3 rd-4 th December 2014, Naples

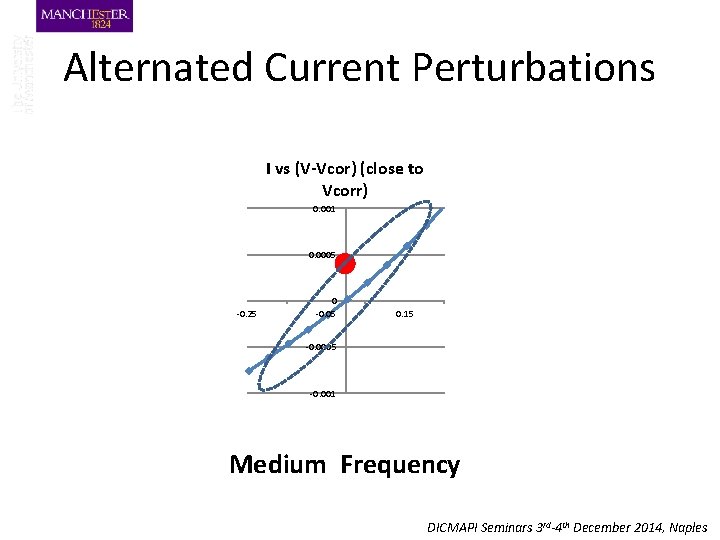
Alternated Current Perturbations I vs (V-Vcor) (close to Vcorr) 0. 001 0. 0005 -0. 25 0 -0. 05 0. 15 -0. 0005 -0. 001 Medium Frequency DICMAPI Seminars 3 rd-4 th December 2014, Naples

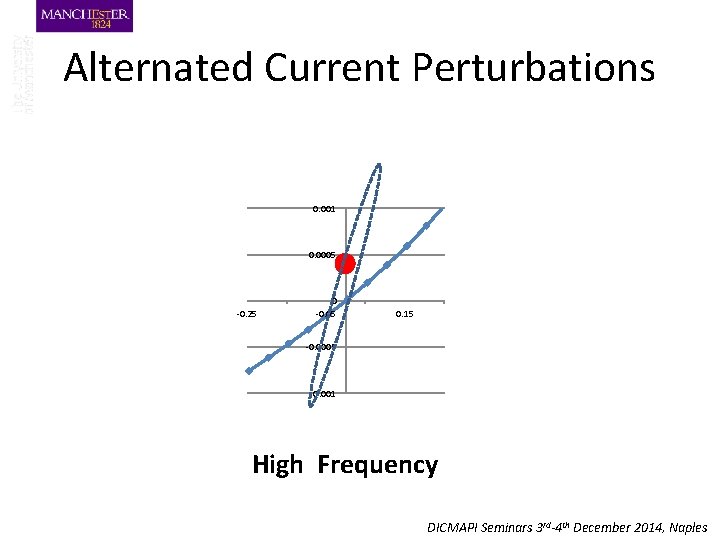
Alternated Current Perturbations 0. 001 0. 0005 -0. 25 0 -0. 05 0. 15 -0. 0005 -0. 001 High Frequency DICMAPI Seminars 3 rd-4 th December 2014, Naples

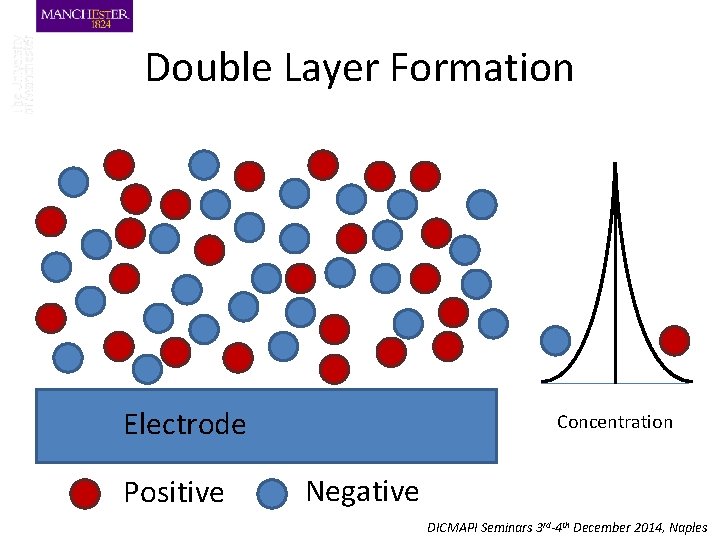
Double Layer Formation Electrode Positive Concentration Negative DICMAPI Seminars 3 rd-4 th December 2014, Naples

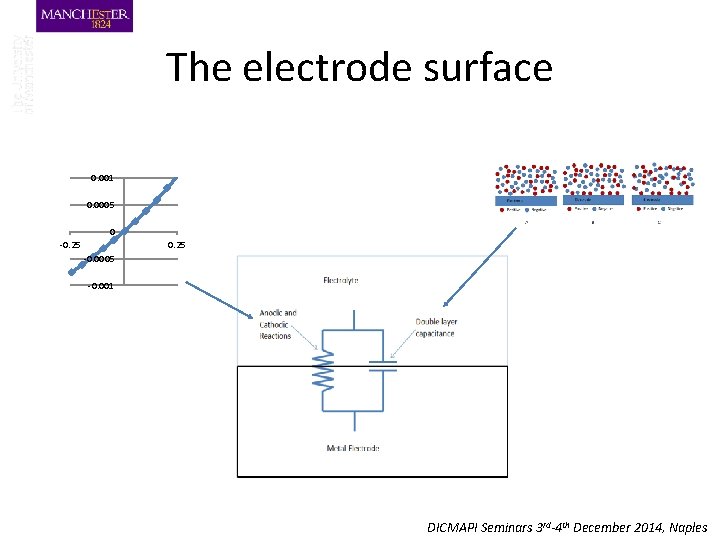
The electrode surface 0. 001 0. 0005 0 -0. 25 -0. 0005 -0. 001 DICMAPI Seminars 3 rd-4 th December 2014, Naples

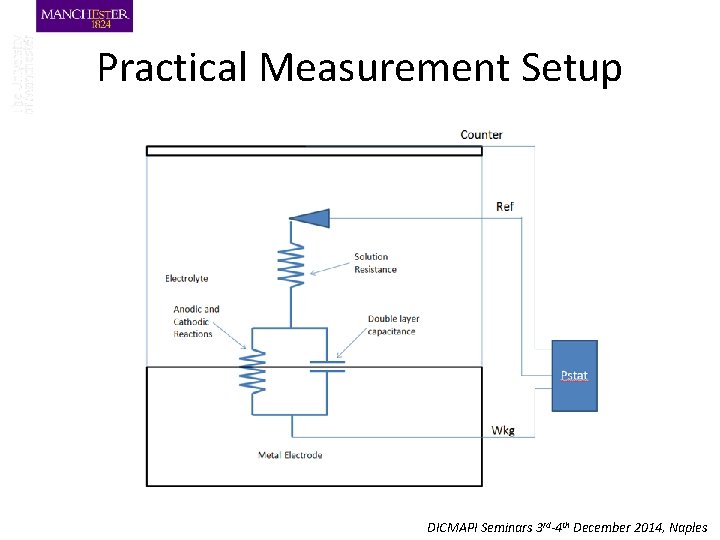
Practical Measurement Setup DICMAPI Seminars 3 rd-4 th December 2014, Naples

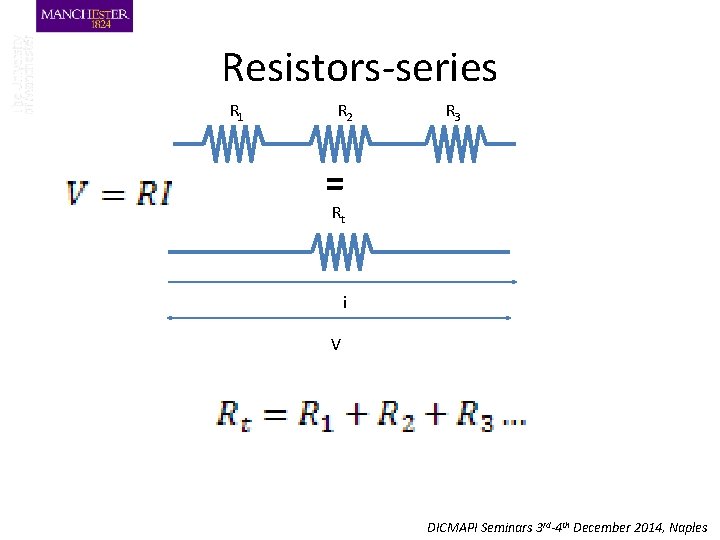
Resistors-series R 1 R 2 R 3 = Rt i V DICMAPI Seminars 3 rd-4 th December 2014, Naples

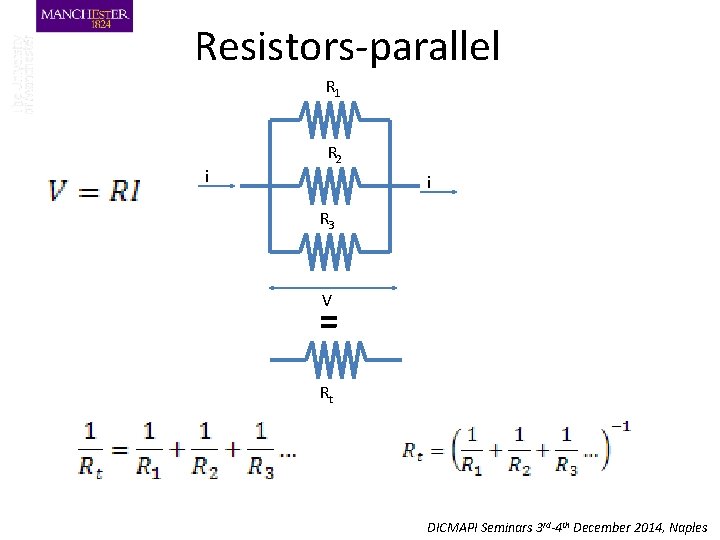
Resistors-parallel R 1 i R 2 i R 3 V = Rt DICMAPI Seminars 3 rd-4 th December 2014, Naples

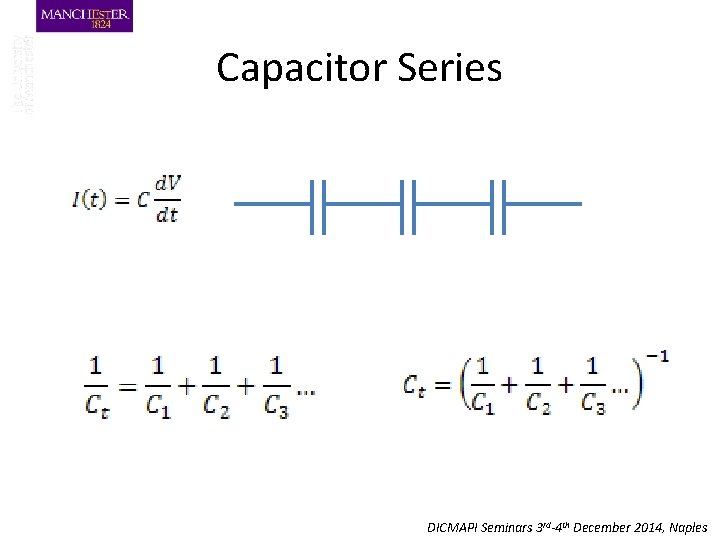
Capacitor Series DICMAPI Seminars 3 rd-4 th December 2014, Naples

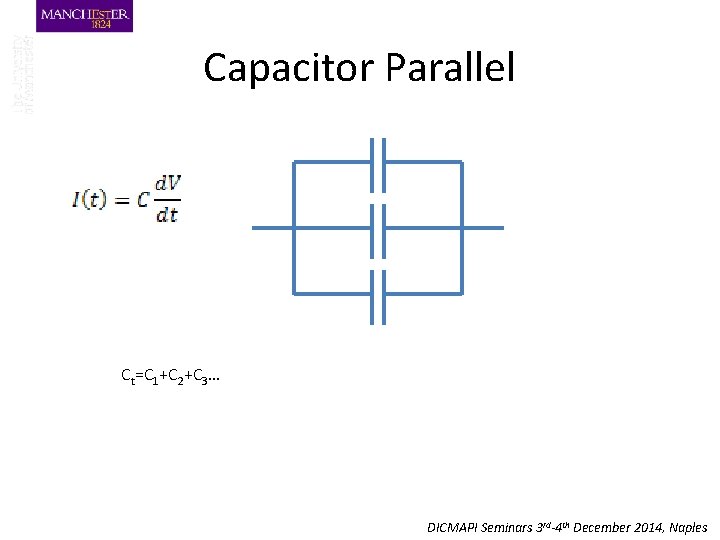
Capacitor Parallel Ct=C 1+C 2+C 3… DICMAPI Seminars 3 rd-4 th December 2014, Naples

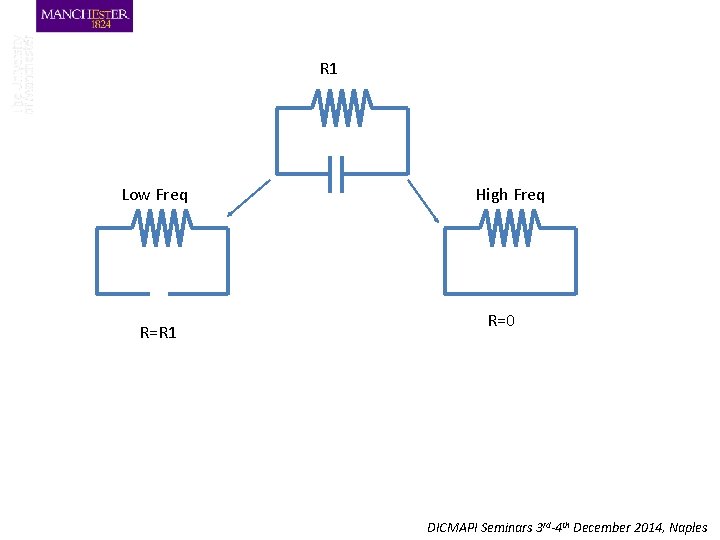
R 1 Low Freq R=R 1 High Freq R=0 DICMAPI Seminars 3 rd-4 th December 2014, Naples

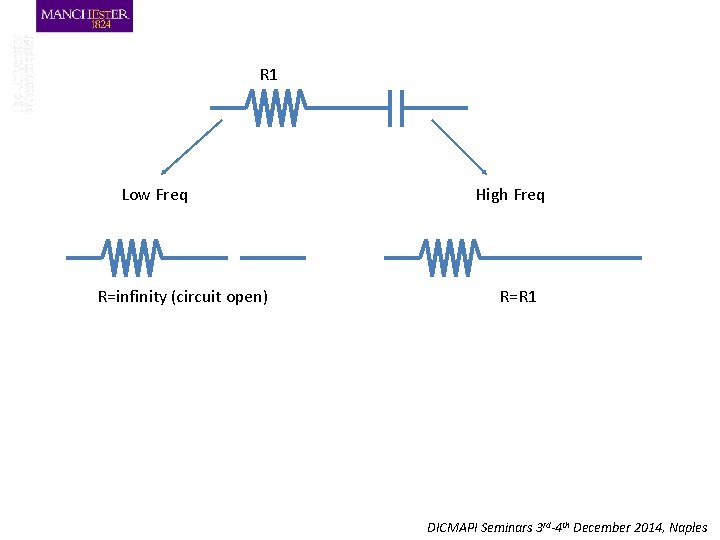
R 1 Low Freq R=infinity (circuit open) High Freq R=R 1 DICMAPI Seminars 3 rd-4 th December 2014, Naples

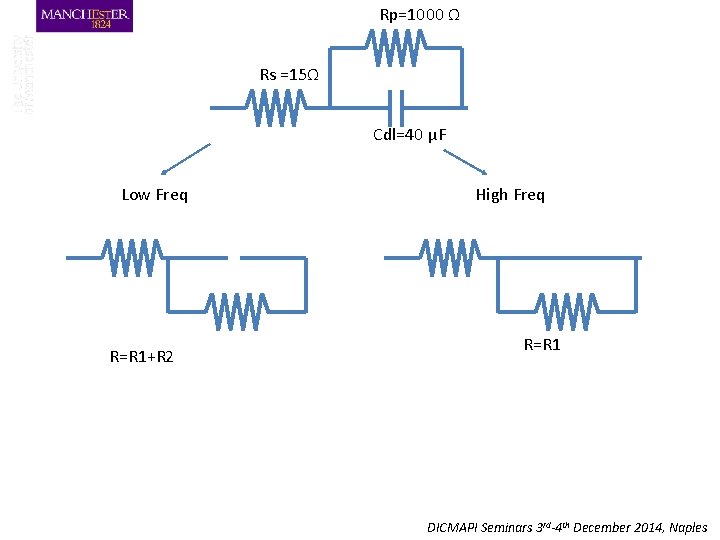
Rp=1000 Ω Rs =15Ω Cdl=40 μF Low Freq R=R 1+R 2 High Freq R=R 1 DICMAPI Seminars 3 rd-4 th December 2014, Naples

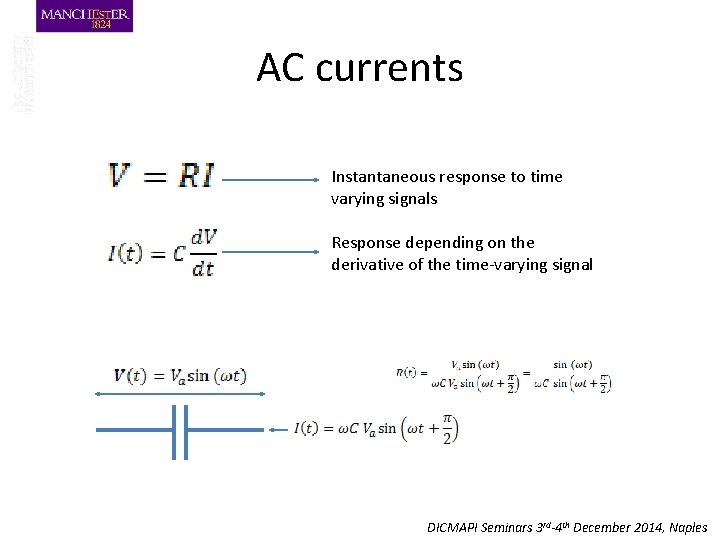
AC currents Instantaneous response to time varying signals Response depending on the derivative of the time-varying signal DICMAPI Seminars 3 rd-4 th December 2014, Naples

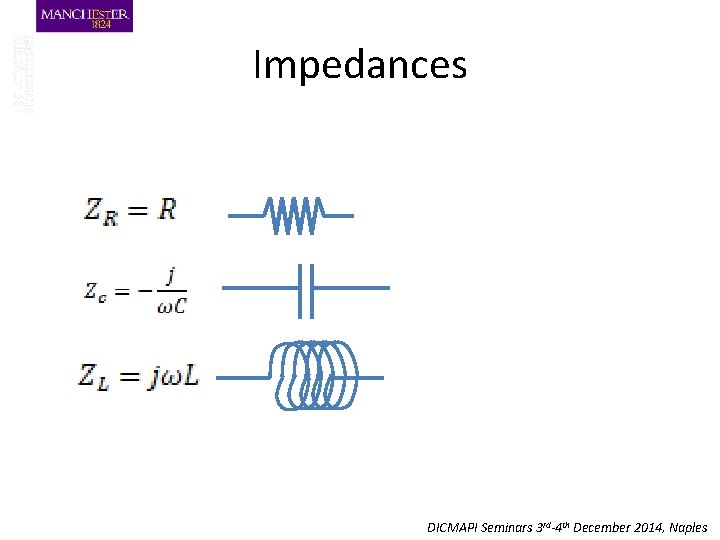
Impedances DICMAPI Seminars 3 rd-4 th December 2014, Naples

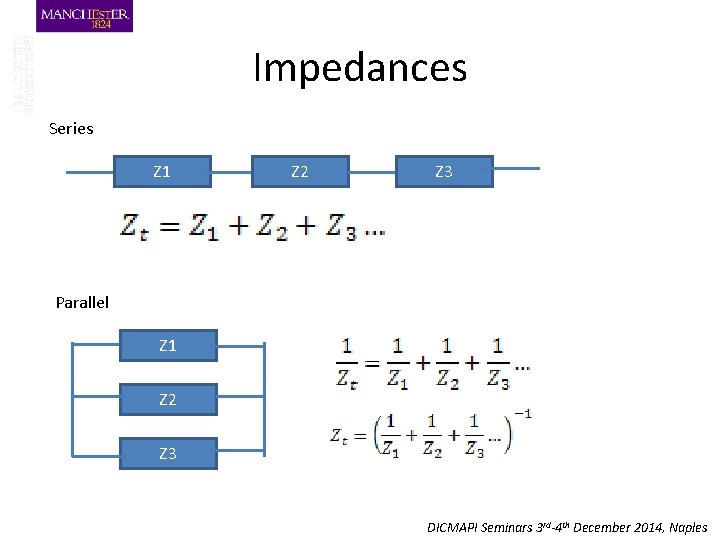
Impedances Series Z 1 Z 2 Z 3 Parallel Z 1 Z 2 Z 3 DICMAPI Seminars 3 rd-4 th December 2014, Naples

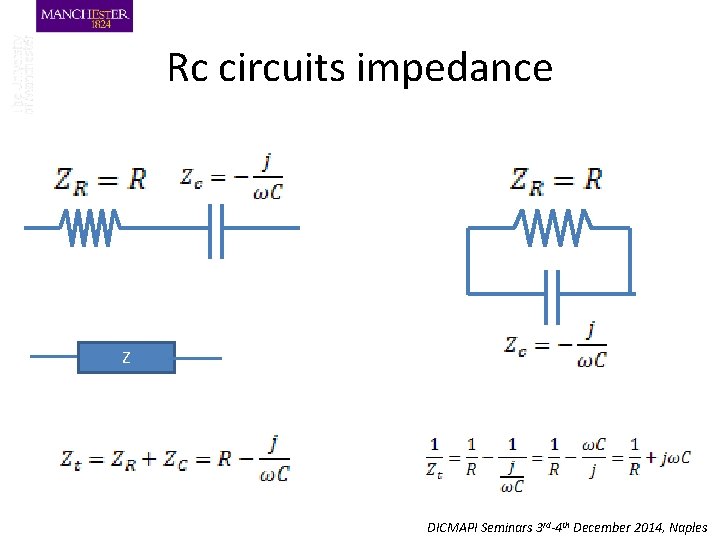
Rc circuits impedance Z DICMAPI Seminars 3 rd-4 th December 2014, Naples

The electrode surface 0. 001 0. 0005 0 -0. 25 -0. 0005 -0. 001 DICMAPI Seminars 3 rd-4 th December 2014, Naples

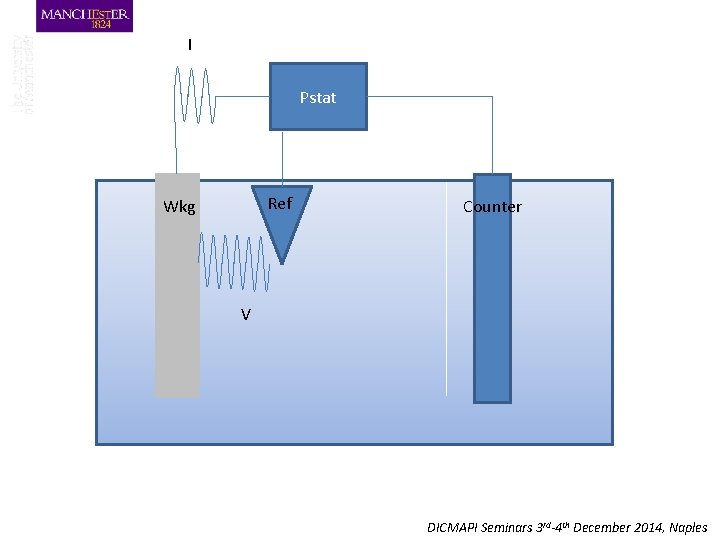
I Pstat Ref Wkg Counter V DICMAPI Seminars 3 rd-4 th December 2014, Naples

Impedance Measurement Modulus and phase at freq 1 Modulus and phase at freq 2 Modulus and phase at freq 3 50 DICMAPI Seminars 3 rd-4 th December 2014, Naples

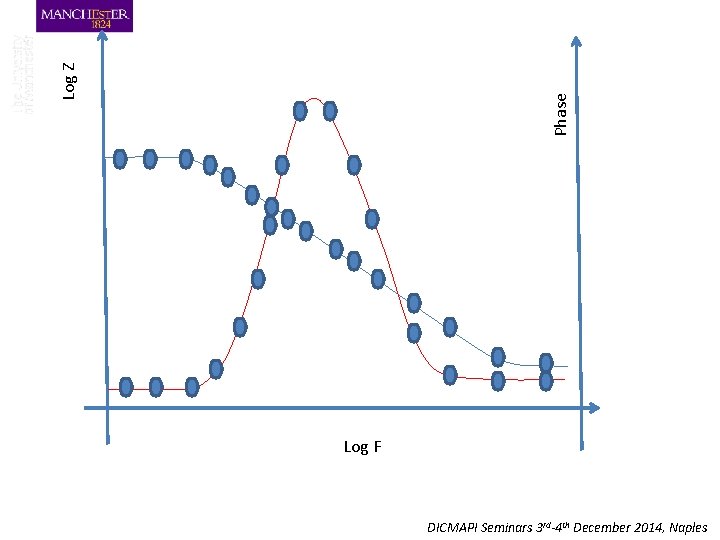
Phase Log Z Log F DICMAPI Seminars 3 rd-4 th December 2014, Naples

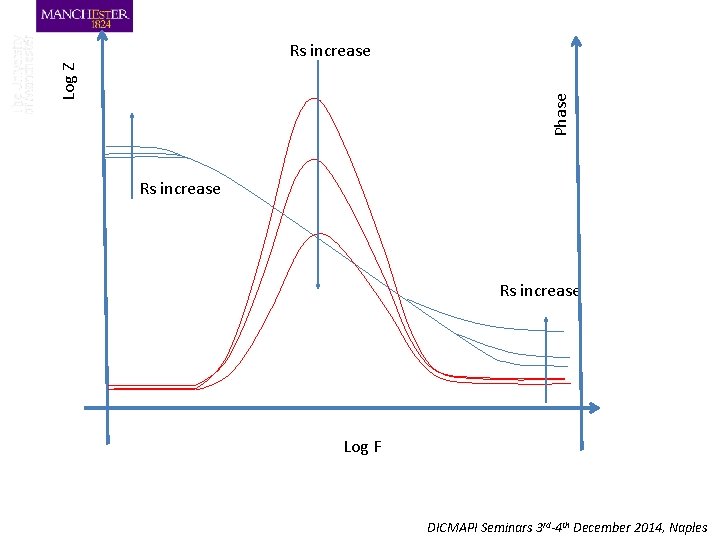
Phase Log Z Rs increase Log F DICMAPI Seminars 3 rd-4 th December 2014, Naples

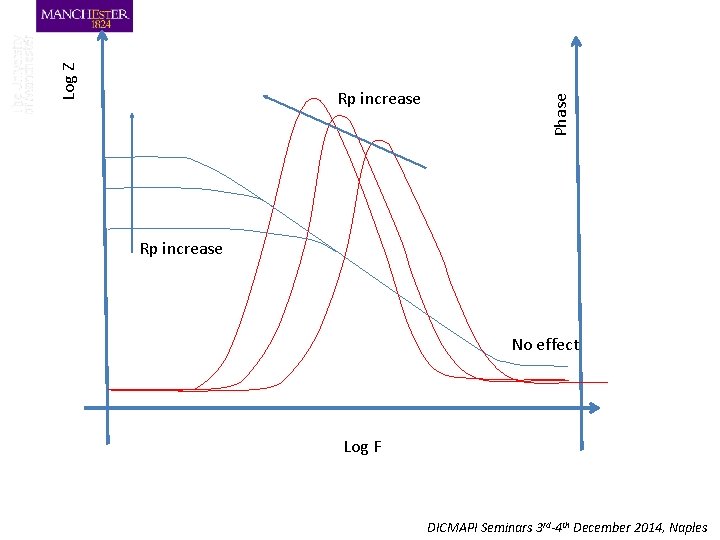
Phase Log Z Rp increase No effect Log F DICMAPI Seminars 3 rd-4 th December 2014, Naples

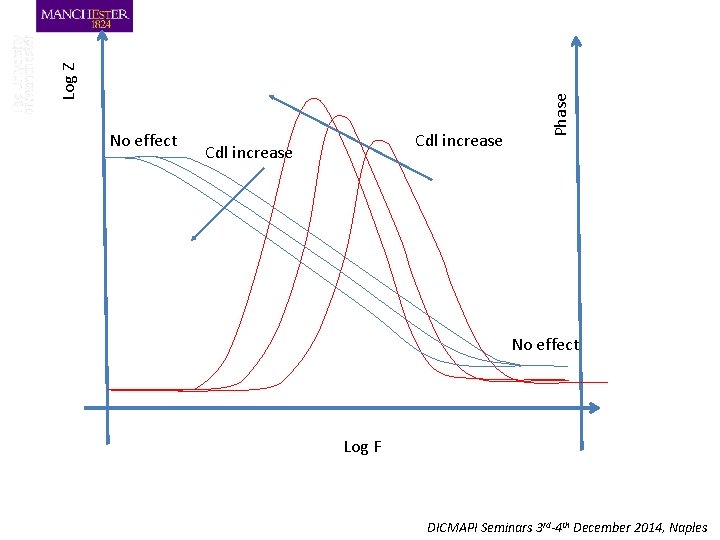
Cdl increase Phase Log Z No effect Log F DICMAPI Seminars 3 rd-4 th December 2014, Naples

DICMAPI Seminars 3 rd-4 th December 2014, Naples

Summary • EIS is based on measuring the current response resulting from the application of a sinusoidal AC signal of variable frequency • EIS is less distructive than DC techniques due to the small signal applied • The simplest circuit representing an electrode is the (R(CR)) • Information on solution resistance, polarization resistance and double layer capacitance can be obtained. Suggested Reading Corrosion Testing Made Easy: Electrochemical Impedance and Noise, R. A. Cottis, S. Turgoose, and R. Newman, NACE, 1999 DICMAPI Seminars 3 rd-4 th December 2014, Naples
 Impedance definition
Impedance definition Electrochemical impedance spectroscopy
Electrochemical impedance spectroscopy Vector impedance meter
Vector impedance meter Quantization noise in pcm
Quantization noise in pcm Cathode vs anode equation
Cathode vs anode equation Advantages of electrochemical machining
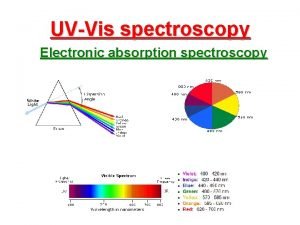
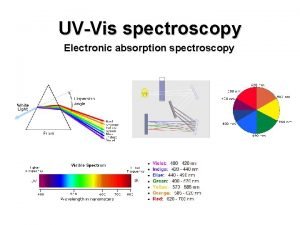
Advantages of electrochemical machining Uv vis spectroscopy in pharmaceutical analysis
Uv vis spectroscopy in pharmaceutical analysis What is admittance in ac circuit
What is admittance in ac circuit Electrochemical deposition
Electrochemical deposition Electrochemical theory of corrosion
Electrochemical theory of corrosion Electrochemical machining animation
Electrochemical machining animation Action potential propagation
Action potential propagation Electrochemical series
Electrochemical series Types of electrochemical sensors
Types of electrochemical sensors The electrochemical series table
The electrochemical series table Diffusion limited
Diffusion limited Difference between dry and wet corrosion
Difference between dry and wet corrosion Dse
Dse What are electrochemical series
What are electrochemical series Electrochemical series order
Electrochemical series order Electrochemical impulse
Electrochemical impulse Differences between nervous system and endocrine
Differences between nervous system and endocrine Types of electrochemical corrosion
Types of electrochemical corrosion The stage that creates an “electrochemical gradient”. *
The stage that creates an “electrochemical gradient”. * How breathalyzer electrochemical
How breathalyzer electrochemical Electrochemical gradient
Electrochemical gradient What is a half reaction
What is a half reaction Electrochemical gradient
Electrochemical gradient Giner electrochemical systems
Giner electrochemical systems Balance redox
Balance redox What is electrochemical
What is electrochemical Electrochemical impulse
Electrochemical impulse Gto thyristor controlled series capacitor
Gto thyristor controlled series capacitor Line current and phase current
Line current and phase current Impedance triangle
Impedance triangle Impedance relay
Impedance relay Earth fault loop impedance
Earth fault loop impedance Intrinsic impedance of good conductor
Intrinsic impedance of good conductor Equation for gain of op amp
Equation for gain of op amp Output impedance of op amp formula
Output impedance of op amp formula Peak reading voltmeter
Peak reading voltmeter Purely resistive circuit
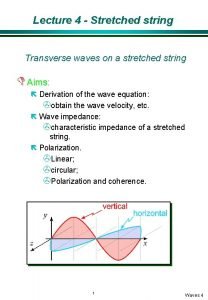
Purely resistive circuit Transverse impedance of a string
Transverse impedance of a string Draw impedance triangle
Draw impedance triangle 163 ent
163 ent Attenuation constant formula
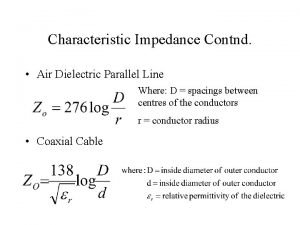
Attenuation constant formula Characteristic impedance definition
Characteristic impedance definition Define intrinsic impedance
Define intrinsic impedance Couplage par impédance commune
Couplage par impédance commune Contnd
Contnd Special diode
Special diode Parallel rc circuit impedance
Parallel rc circuit impedance Per unit formula in power system
Per unit formula in power system Scattering matrix
Scattering matrix Line
Line Impedance rlc
Impedance rlc Y=1/z impedance
Y=1/z impedance