ELECTROCHEMICAL CELLS Voltaic Cell Volat construct its cell

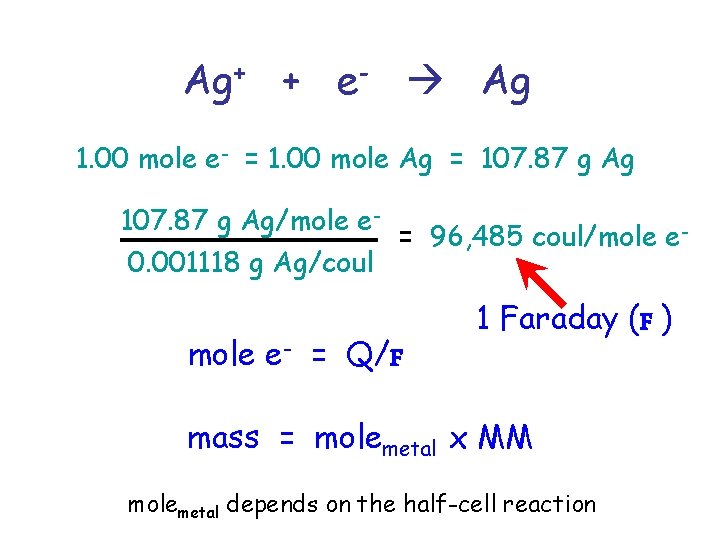

- Slides: 16

ELECTROCHEMICAL CELLS Voltaic Cell Volat construct its cell by the electricity occured due to the attraction between metal pairs.

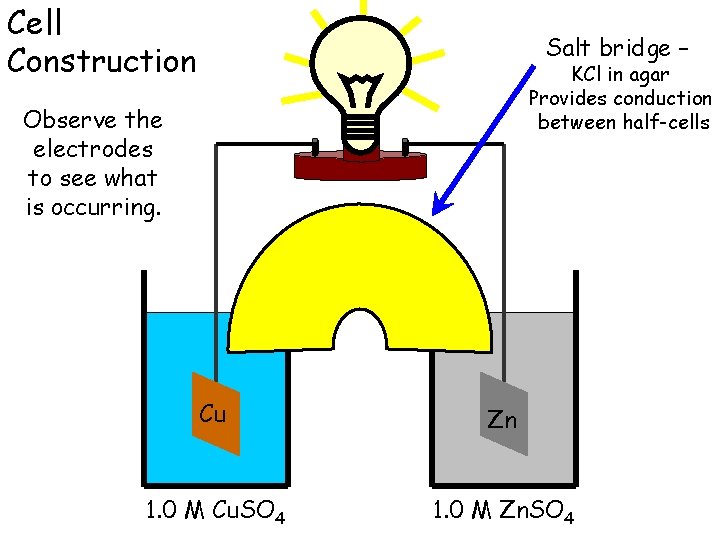
Cell Construction Salt bridge – KCl in agar Provides conduction between half-cells Observe the electrodes to see what is occurring. Cu Zn 1. 0 M Cu. SO 4 1. 0 M Zn. SO 4

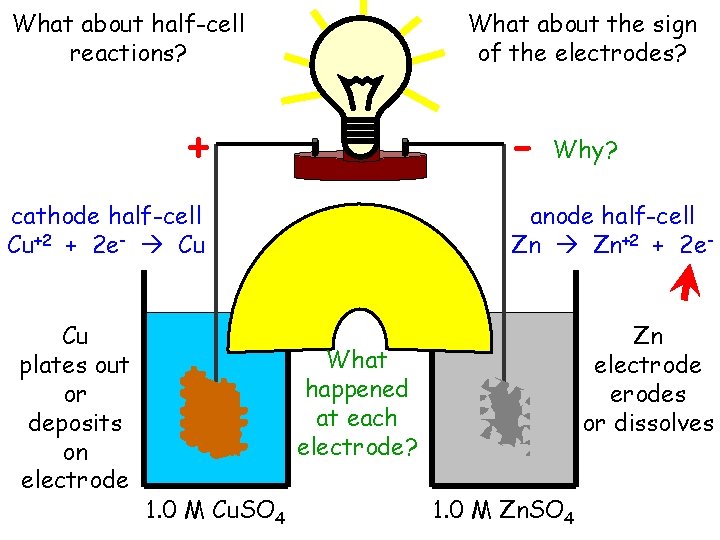
What about half-cell reactions? What about the sign of the electrodes? - + cathode half-cell Cu+2 + 2 e- Cu Cu plates out or deposits on electrode Cu 1. 0 M Cu. SO 4 Why? anode half-cell Zn Zn+2 + 2 e- What happened at each electrode? Zn 1. 0 M Zn. SO 4 Zn electrode erodes or dissolves

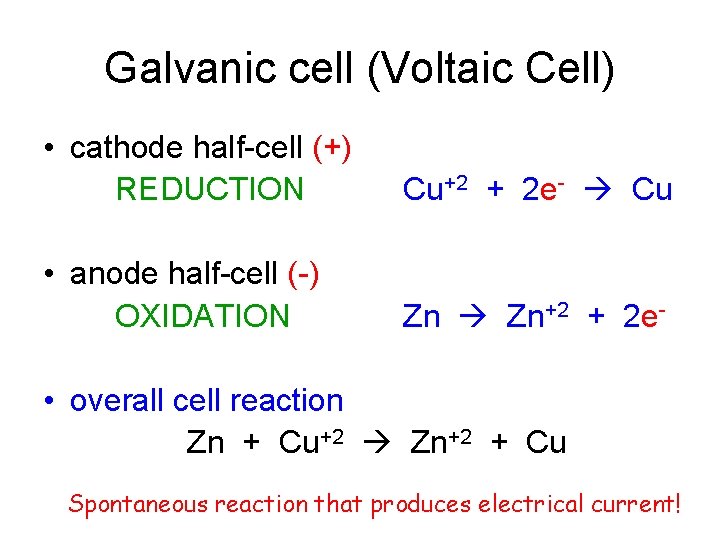
Galvanic cell (Voltaic Cell) • cathode half-cell (+) REDUCTION Cu+2 + 2 e- Cu • anode half-cell (-) OXIDATION Zn Zn+2 + 2 e- • overall cell reaction Zn + Cu+2 Zn+2 + Cu Spontaneous reaction that produces electrical current!


ELECTROLYSIS

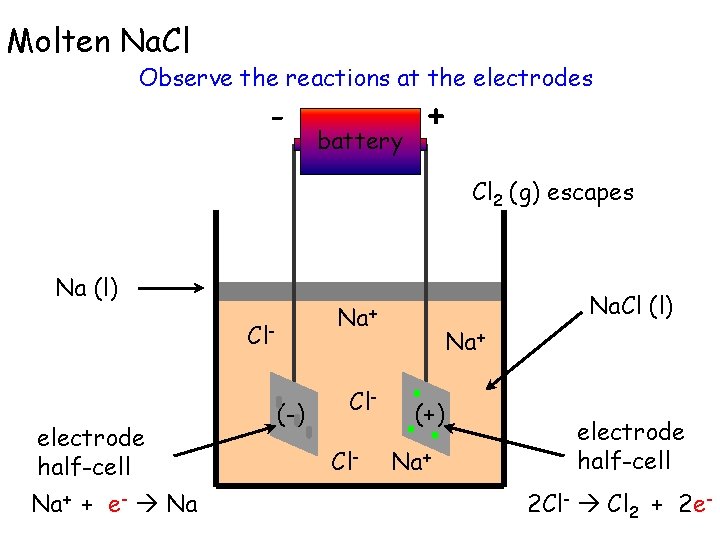
Molten Na. Cl Observe the reactions at the electrodes - battery + Cl 2 (g) escapes Na (l) Cl- electrode half-cell Na+ + e- Na Na. Cl (l) Na+ (-) Cl. Cl- Na+ (+) Na+ electrode half-cell 2 Cl- Cl 2 + 2 e-

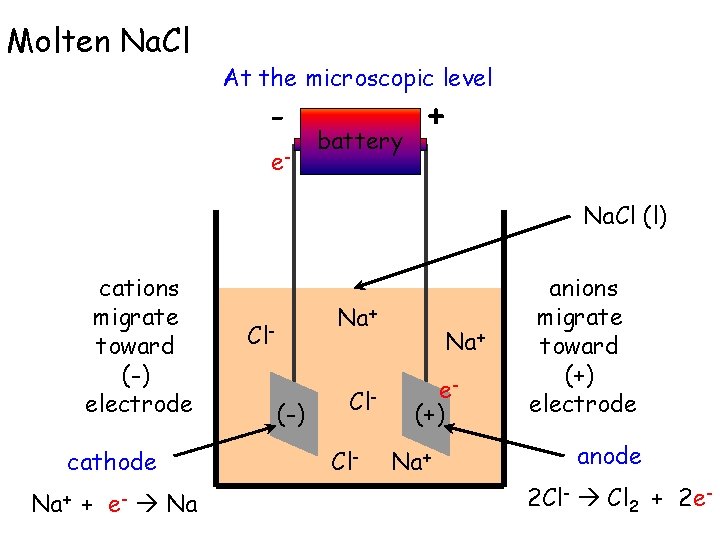
Molten Na. Cl At the microscopic level - e- battery + Na. Cl (l) cations migrate toward (-) electrode cathode Na+ + e- Na Na+ Cl(-) Cl. Cl- Na+ e(+) Na+ anions migrate toward (+) electrode anode 2 Cl- Cl 2 + 2 e-

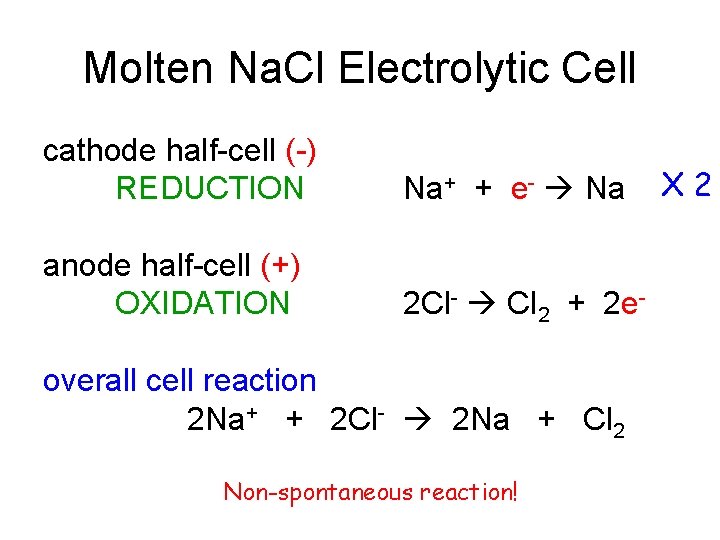
Molten Na. Cl Electrolytic Cell cathode half-cell (-) REDUCTION Na+ + e- Na anode half-cell (+) OXIDATION 2 Cl- Cl 2 + 2 e- overall cell reaction 2 Na+ + 2 Cl- 2 Na + Cl 2 Non-spontaneous reaction! X 2

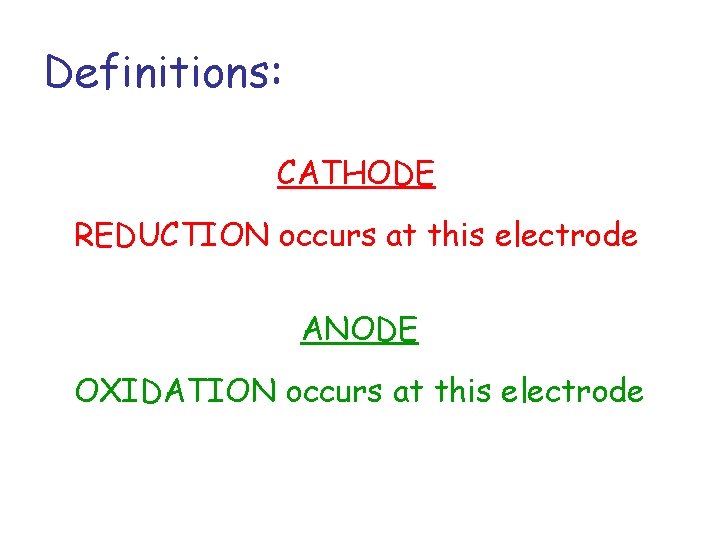
Definitions: CATHODE REDUCTION occurs at this electrode ANODE OXIDATION occurs at this electrode

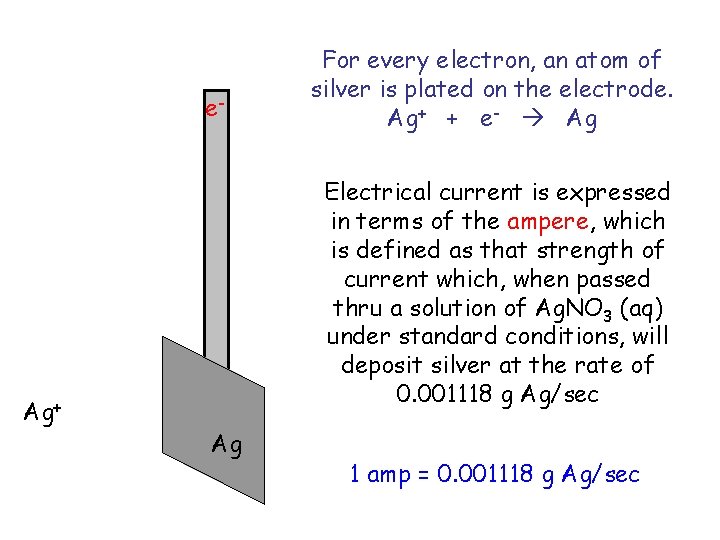
e- Ag+ For every electron, an atom of silver is plated on the electrode. Ag+ + e- Ag Electrical current is expressed in terms of the ampere, which is defined as that strength of current which, when passed thru a solution of Ag. NO 3 (aq) under standard conditions, will deposit silver at the rate of 0. 001118 g Ag/sec Ag 1 amp = 0. 001118 g Ag/sec

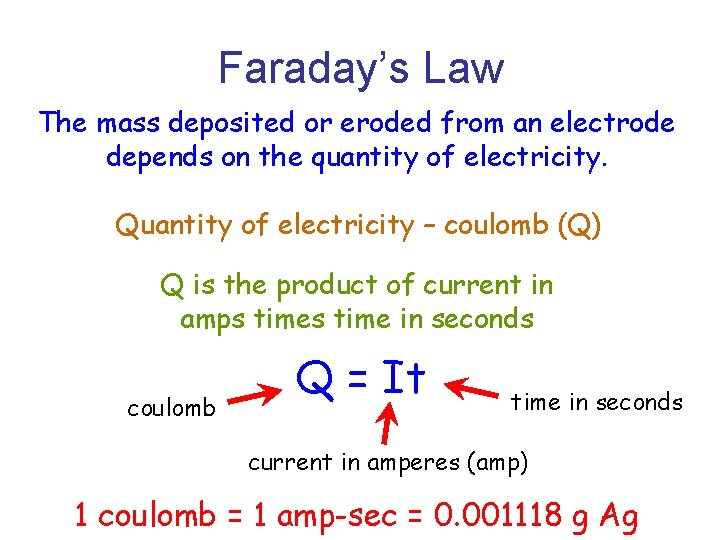
Faraday’s Law The mass deposited or eroded from an electrode depends on the quantity of electricity. Quantity of electricity – coulomb (Q) Q is the product of current in amps time in seconds coulomb Q = It time in seconds current in amperes (amp) 1 coulomb = 1 amp-sec = 0. 001118 g Ag
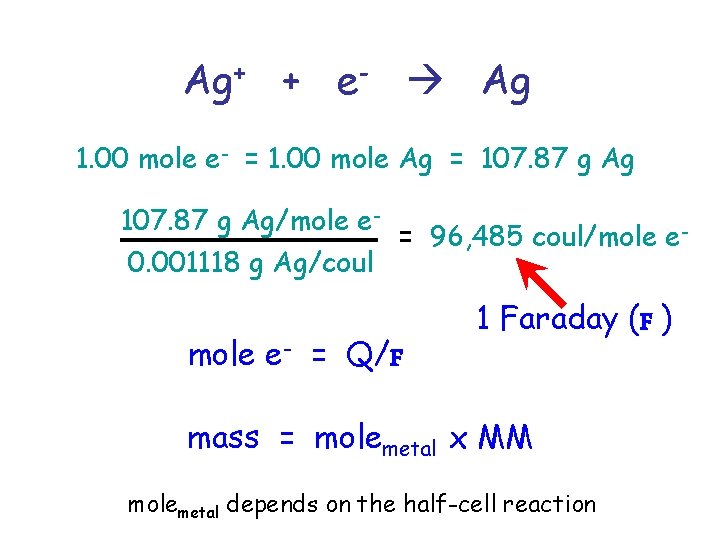
Ag+ + e- Ag 1. 00 mole e- = 1. 00 mole Ag = 107. 87 g Ag/mole e= 96, 485 coul/mole e 0. 001118 g Ag/coul mole e- = Q/F 1 Faraday (F ) mass = molemetal x MM molemetal depends on the half-cell reaction

l. Faraday states that the atom can just carry certain amounts of or the simple multiples of certain amounts of electric current. l. This concludes that electric current can be carried by certain chunks. l. This shows electricity is composed of particles.

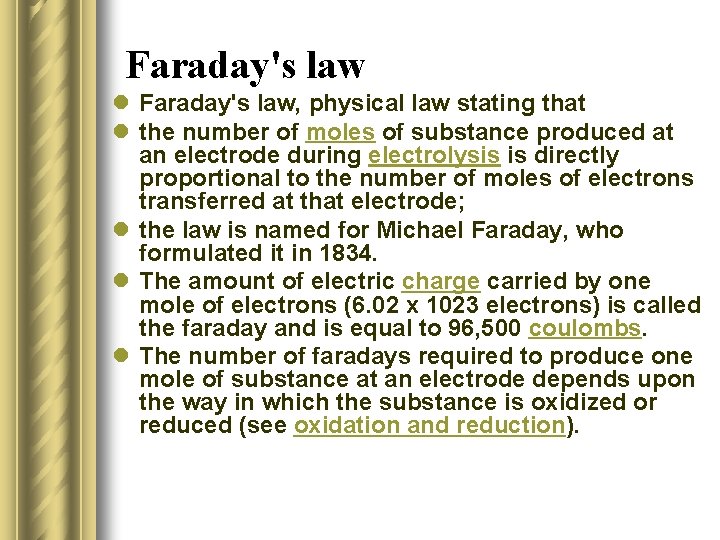
Faraday's law l Faraday's law, physical law stating that l the number of moles of substance produced at an electrode during electrolysis is directly proportional to the number of moles of electrons transferred at that electrode; l the law is named for Michael Faraday, who formulated it in 1834. l The amount of electric charge carried by one mole of electrons (6. 02 x 1023 electrons) is called the faraday and is equal to 96, 500 coulombs. l The number of faradays required to produce one mole of substance at an electrode depends upon the way in which the substance is oxidized or reduced (see oxidation and reduction).

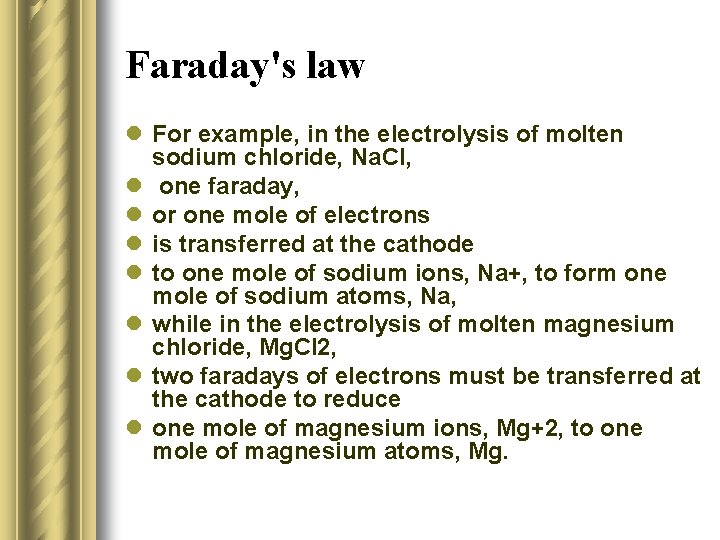
Faraday's law l For example, in the electrolysis of molten sodium chloride, Na. Cl, l one faraday, l or one mole of electrons l is transferred at the cathode l to one mole of sodium ions, Na+, to form one mole of sodium atoms, Na, l while in the electrolysis of molten magnesium chloride, Mg. Cl 2, l two faradays of electrons must be transferred at the cathode to reduce l one mole of magnesium ions, Mg+2, to one mole of magnesium atoms, Mg.