Electricity Magnetism Part I Static Electricity Electric Fields
















- Slides: 57

Electricity & Magnetism Part I – Static Electricity & Electric Fields

Table of Contents Electrical Force & Charge Tolman –Stewart Experiment Charging by Conduction v. Induction Charge Polarization Coulomb’s Law – Calculating Electric Force Electric Fields Work & Electric Potential Difference Millikan’s Oil Drop Experiment Capacitors

The Four Fundamental Forces of Nature Gravitational Force – the attraction between two objects due to their masses. (This is the only fundamental force of nature we’ve actually studied in this class – so far!) Electromagnetic Force – what we will be studying starting today! Strong Nuclear Force – The force that holds atomic nuclei together Weak Nuclear Force – Force which kicks in during radioactive decay

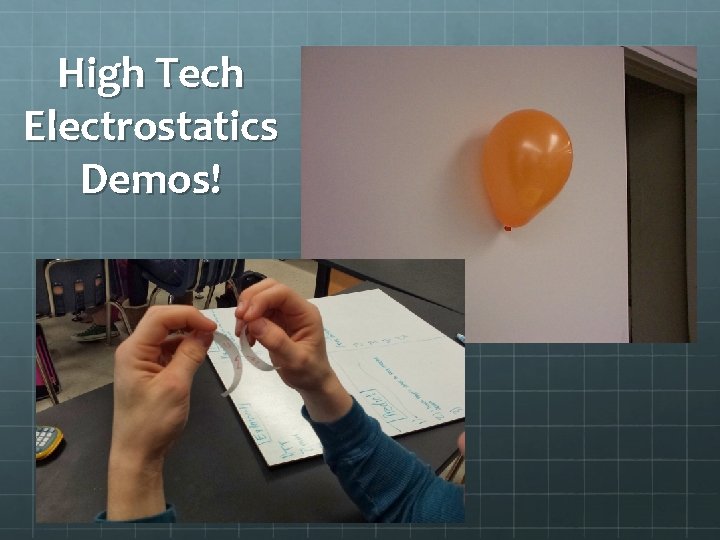
High Tech Electrostatics Demos!

Scotch Tape Demo Get two pieces of Scotch tape, each about 6 -8 inches long. Fold over a half-inch or so on the end so you have a nonsticky “handle” on the end. Stick one of the pieces of tape to a flat, easy-to-peelaway-from surface. (A binder would work well) Stick the second piece of tape right on top of the first piece.

Scotch Tape Demo While the top piece of tape is still attached to it, peel the bottom piece of tape away from the binder or whatever surface you stuck it to. Once fully free from the surface, peel the two pieces of of tape away from each other. Then bring them near each other…

They attract!

Scotch Tape Demo Now stick your pieces of tape back to your binder or other flat surface, except this time put them beside each other – not one atop the other, so that both are stuck to the surface. Then peel them both off. Then bring them near each other…

They repel!

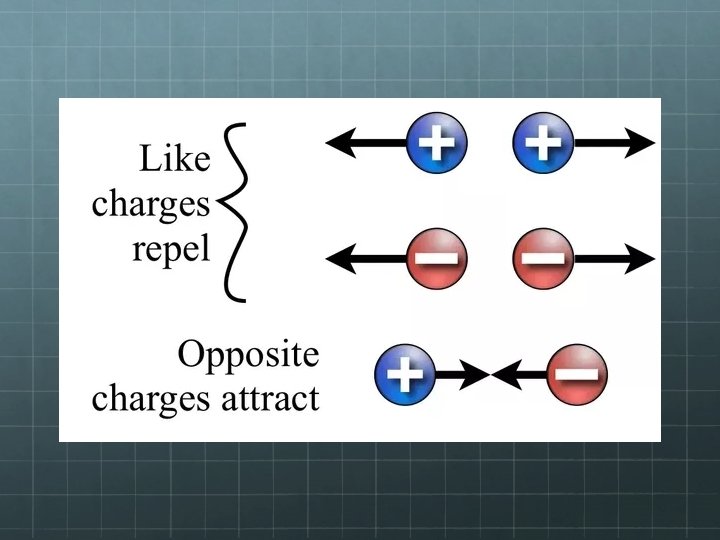
Electric Force & Charge While gravity can only attract, electrical forces can attract and repel. To feel, or exert, gravitational force, an object must possess mass. There’s only one kind of mass, so when two masses are brought together, there’s only one thing for them to do: attract. To feel, or exert, an electrical force, an object must possess what we call charge. Since electrical charges can attract or repel, there must be more than one way for charges to behave around each other. Conclusion – There must be two different kinds of charge! We label these two charges positive & negative.


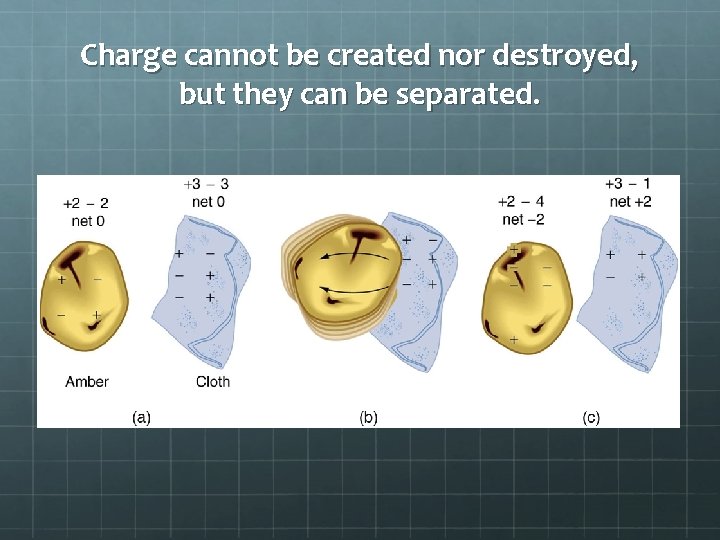
Charge cannot be created nor destroyed, but they can be separated.

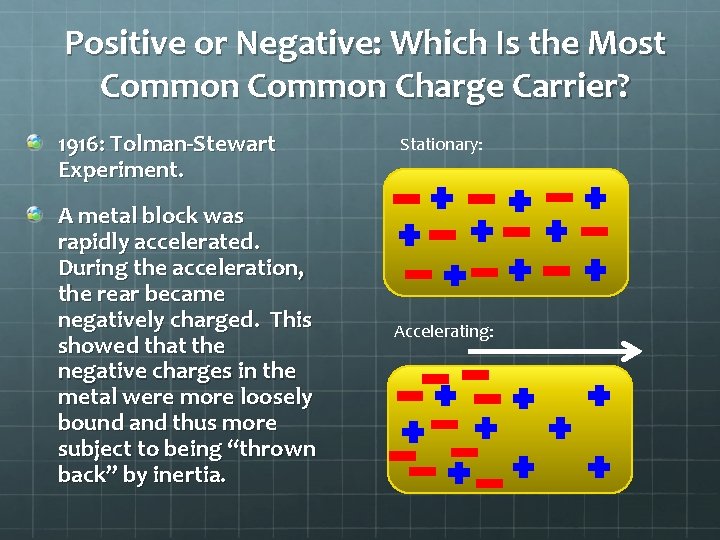
Positive or Negative: Which Is the Most Common Charge Carrier? 1916: Tolman-Stewart Experiment. A metal block was rapidly accelerated. During the acceleration, the rear became negatively charged. This showed that the negative charges in the metal were more loosely bound and thus more subject to being “thrown back” by inertia. Stationary: Accelerating:

The Electroscope: Demonstrating Conduction, Induction, & Charge Polarization

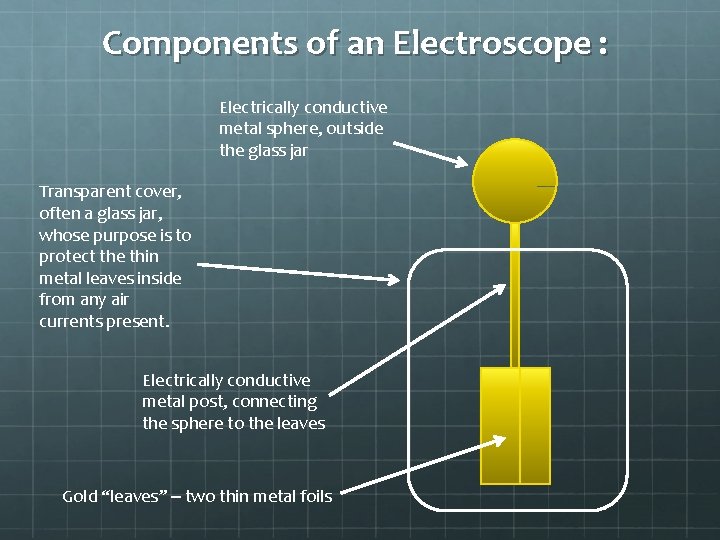
Components of an Electroscope : Electrically conductive metal sphere, outside the glass jar Transparent cover, often a glass jar, whose purpose is to protect the thin metal leaves inside from any air currents present. Electrically conductive metal post, connecting the sphere to the leaves Gold “leaves” – two thin metal foils

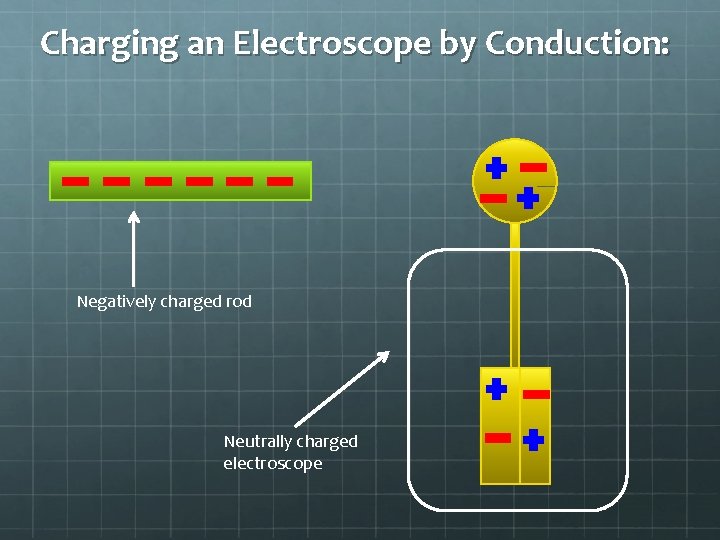
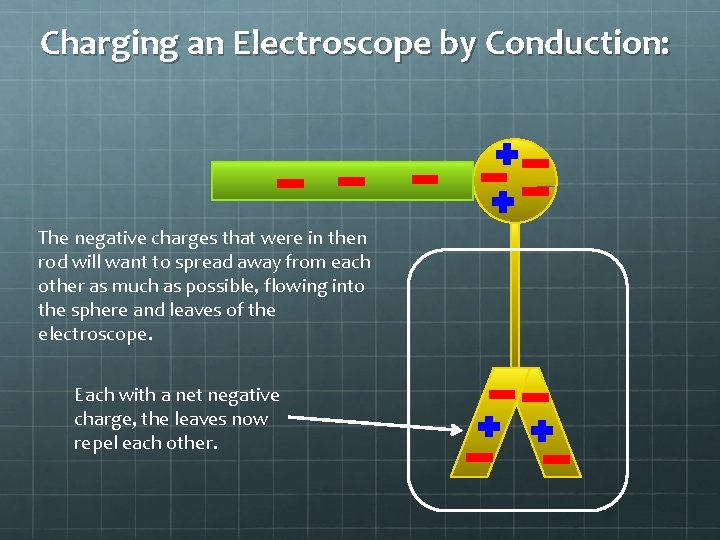
Charging an Electroscope by Conduction: Negatively charged rod Neutrally charged electroscope

Charging an Electroscope by Conduction: Touch the rod to the sphere:

Charging an Electroscope by Conduction: The negative charges that were in then rod will want to spread away from each other as much as possible, flowing into the sphere and leaves of the electroscope. Each with a net negative charge, the leaves now repel each other.

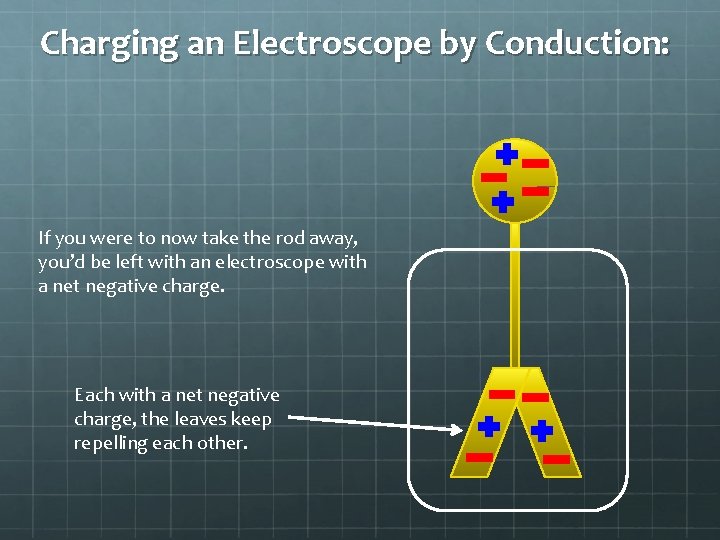
Charging an Electroscope by Conduction: If you were to now take the rod away, you’d be left with an electroscope with a net negative charge. Each with a net negative charge, the leaves keep repelling each other.

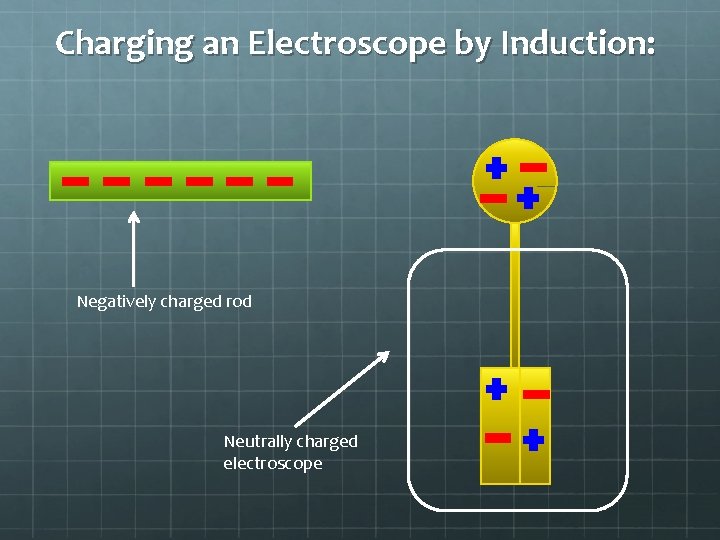
Charging an Electroscope by Induction: Negatively charged rod Neutrally charged electroscope

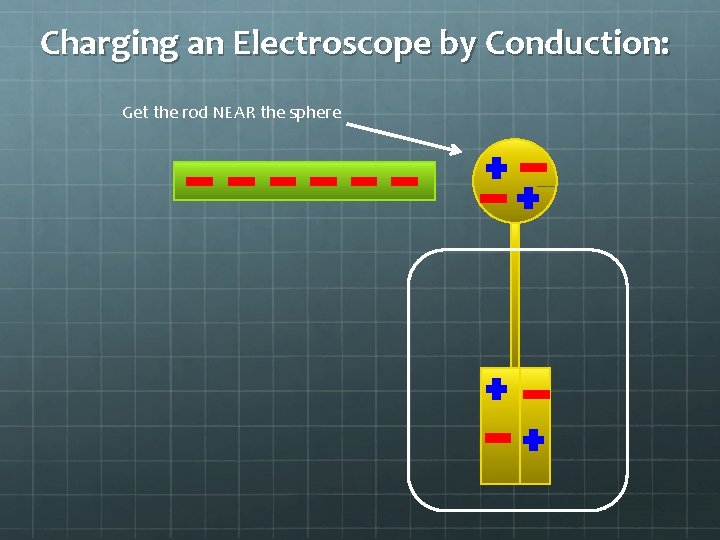
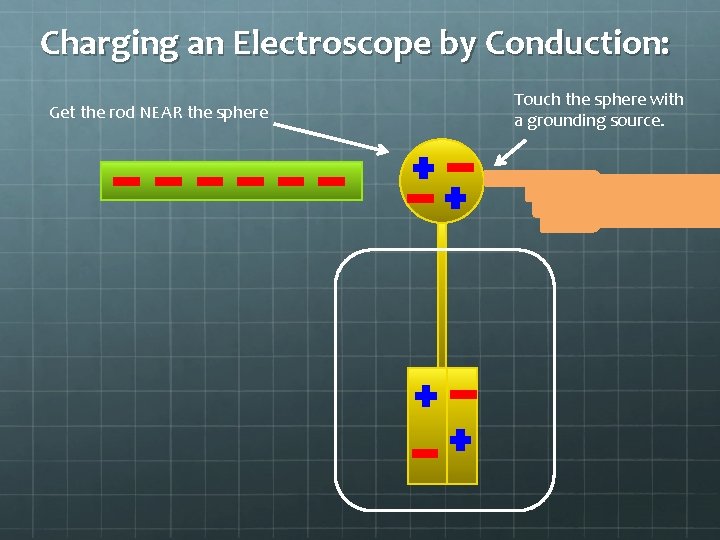
Charging an Electroscope by Conduction: Get the rod NEAR the sphere

Charging an Electroscope by Conduction: Get the rod NEAR the sphere Touch the sphere with a grounding source.

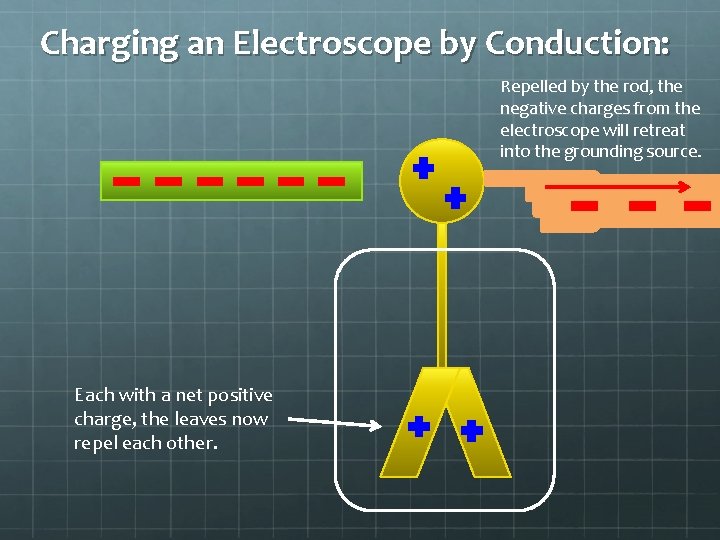
Charging an Electroscope by Conduction: Repelled by the rod, the negative charges from the electroscope will retreat into the grounding source. Each with a net positive charge, the leaves now repel each other.

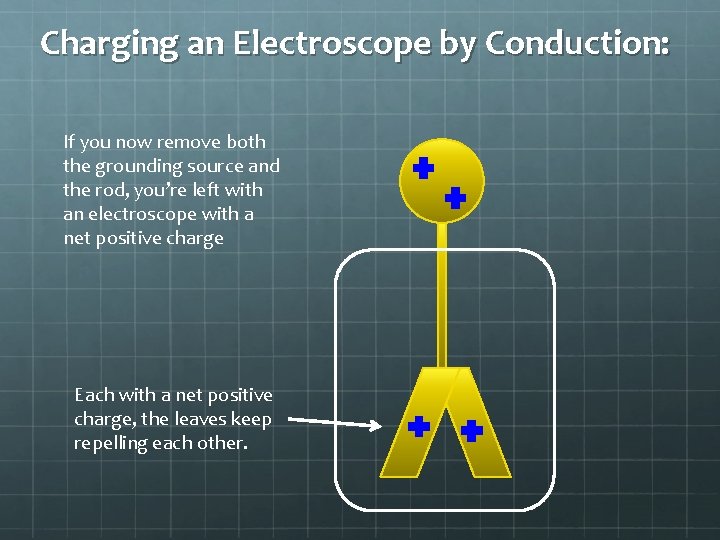
Charging an Electroscope by Conduction: If you now remove both the grounding source and the rod, you’re left with an electroscope with a net positive charge Each with a net positive charge, the leaves keep repelling each other.

Conduction v. Induction Note: Conduction involves actual contact with a charged object; the charged object takes on the same charge as the charging object. Induction does not involve actual contact with a charged object; the charged object takes on the opposite charge as the charging object.

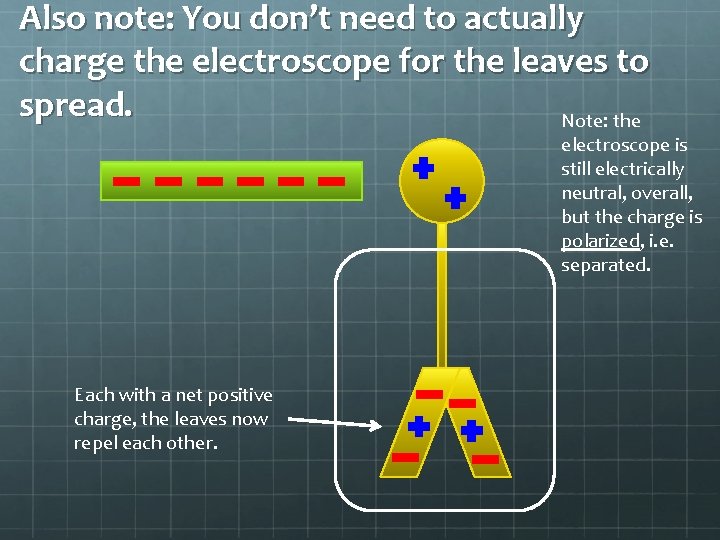
Also note: You don’t need to actually charge the electroscope for the leaves to spread. Note: the electroscope is still electrically neutral, overall, but the charge is polarized, i. e. separated. Each with a net positive charge, the leaves now repel each other.

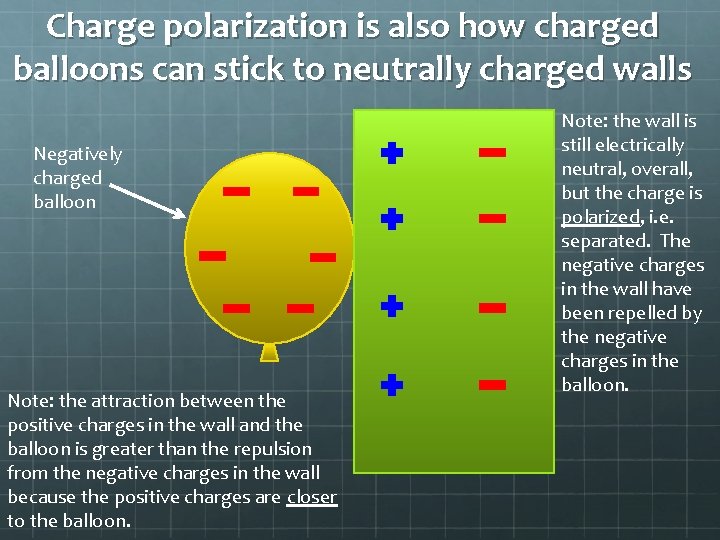
Charge polarization is also how charged balloons can stick to neutrally charged walls Negatively charged balloon Note: the attraction between the positive charges in the wall and the balloon is greater than the repulsion from the negative charges in the wall because the positive charges are closer to the balloon. Note: the wall is still electrically neutral, overall, but the charge is polarized, i. e. separated. The negative charges in the wall have been repelled by the negative charges in the balloon.

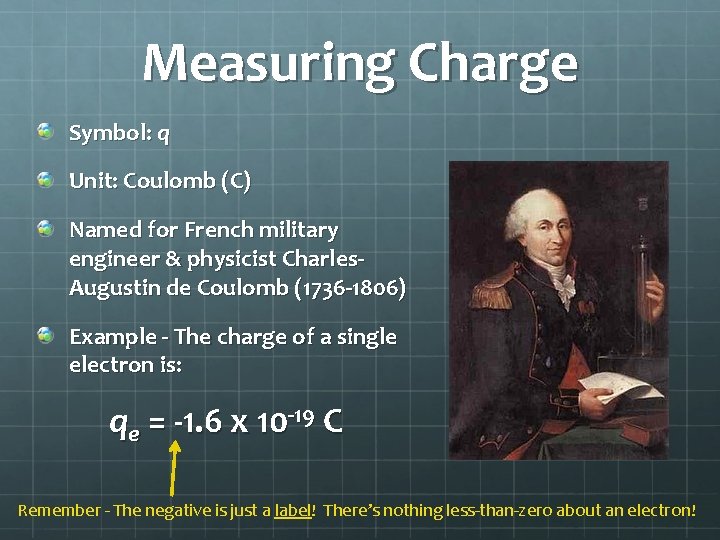
Measuring Charge Symbol: q Unit: Coulomb (C) Named for French military engineer & physicist Charles. Augustin de Coulomb (1736 -1806) Example - The charge of a single electron is: qe = -1. 6 x 10 -19 C Remember - The negative is just a label! There’s nothing less-than-zero about an electron!

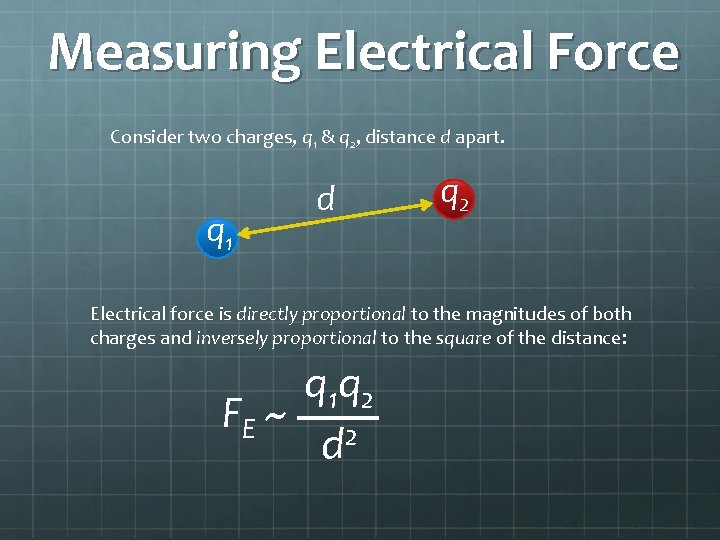
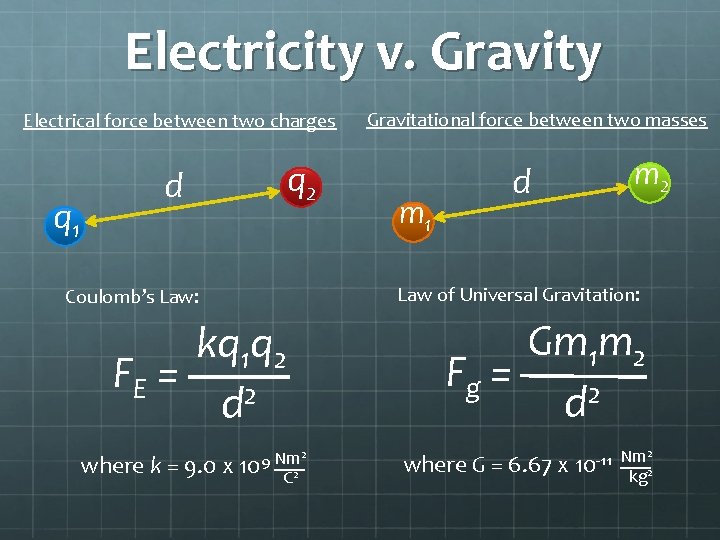
Measuring Electrical Force Consider two charges, q 1 & q 2, distance d apart. q 1 d q 2 Electrical force is directly proportional to the magnitudes of both charges and inversely proportional to the square of the distance: q 1 q 2 FE ~ 2 d

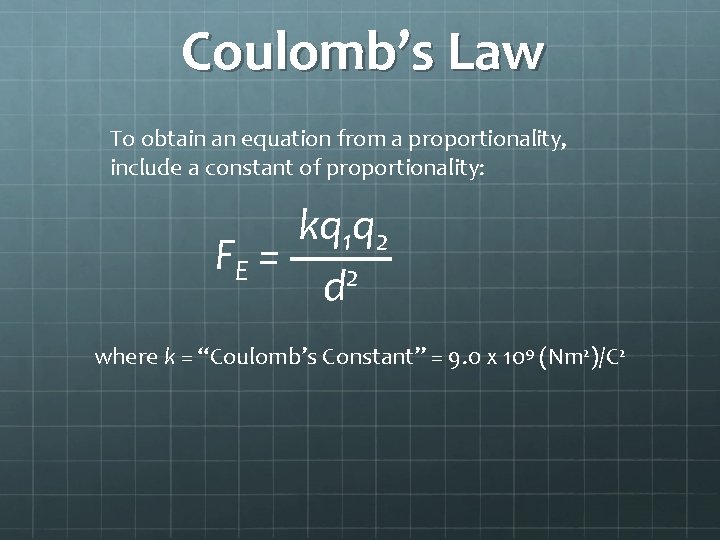
Coulomb’s Law To obtain an equation from a proportionality, include a constant of proportionality: kq 1 q 2 FE = 2 d where k = “Coulomb’s Constant” = 9. 0 x 109 (Nm 2)/C 2

Electricity v. Gravity Electrical force between two charges q 1 q 2 d m 1 d m 2 Law of Universal Gravitation: Coulomb’s Law: kq 1 q 2 FE = 2 d where k = 9. 0 x Gravitational force between two masses Nm 2 9 10 C 2 Gm 1 m 2 Fg = d 2 where G = 6. 67 x 10 -11 Nm 2 kg 2

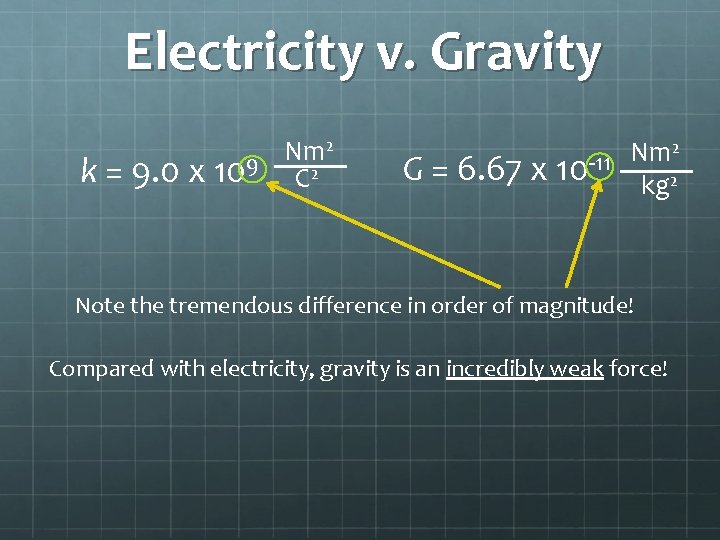
Electricity v. Gravity 2 Nm k = 9. 0 x 109 C 2 2 Nm G = 6. 67 x 10 -11 kg 2 Note the tremendous difference in order of magnitude! Compared with electricity, gravity is an incredibly weak force!

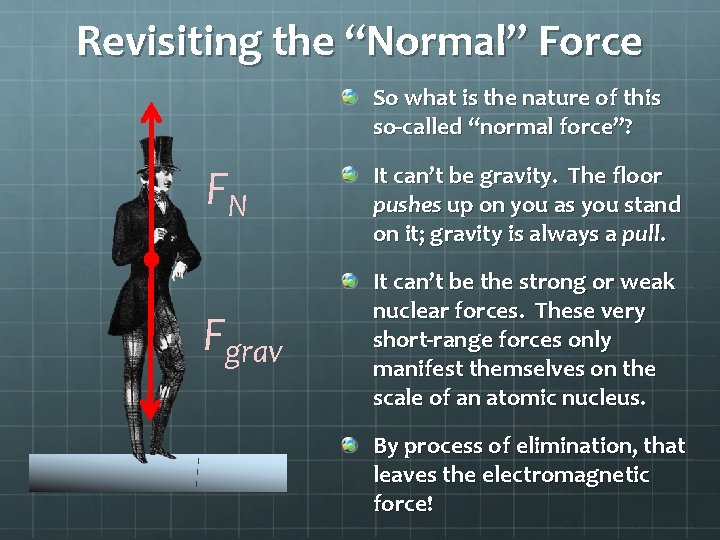
Revisiting the “Normal” Force So what is the nature of this so-called “normal force”? FN Fgrav It can’t be gravity. The floor pushes up on you as you stand on it; gravity is always a pull. It can’t be the strong or weak nuclear forces. These very short-range forces only manifest themselves on the scale of an atomic nucleus. By process of elimination, that leaves the electromagnetic force!

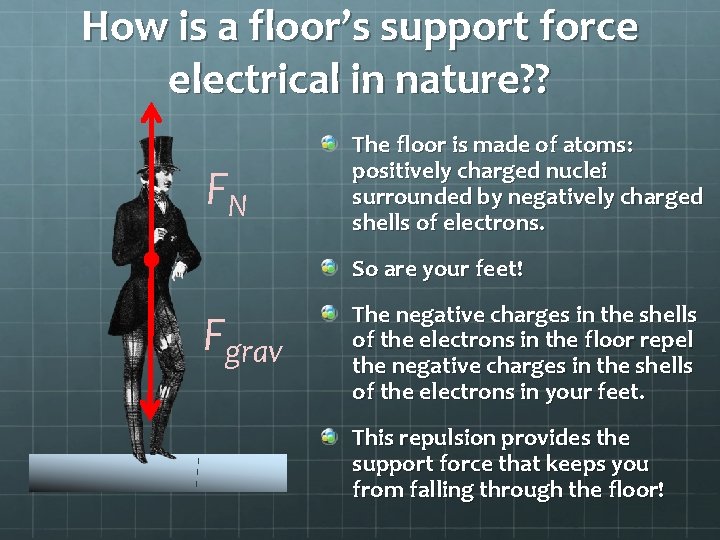
How is a floor’s support force electrical in nature? ? FN The floor is made of atoms: positively charged nuclei surrounded by negatively charged shells of electrons. So are your feet! Fgrav The negative charges in the shells of the electrons in the floor repel the negative charges in the shells of the electrons in your feet. This repulsion provides the support force that keeps you from falling through the floor!

How does this demonstrate that gravity is weak? Anytime two solid objects fail to pass through each other like ghosts, it is due to electric repulsion. This is evident any time any two solid objects come in to contact. With gravity, forces between any two objects is negligible unless at least one of those objects is HUGE. (This is why you don’t feel compelled to lean toward the street every time a large truck drives by!) Here’s another example: If I’m holding a pen in my hand right now, then the entire planet Earth is pulling with ALL of its gravity, trying to make that pen fall. Yet the pen isn’t falling. Why? Because I am overcoming the gravitational pull of our entire planet with literally nothing more than an handful of

Force Fields Recall, a “force field” is a region of space where, if you possess the right property, you feel a force. Force field strength is defined as the amount of force per unit quantity of the property that lets you feel that force. Consider, for example, a gravitational field caused by a large mass M and felt by small test mass m. Gravitational field strength (denoted g) = Fg/m But Fg = (GMm)/d 2 So g = [(GMm)/d 2]/m = (GM)/d 2 Note: g doesn’t depend on the small test mass m.

Electric Fields Similarly, consider an electric field caused by some charge Q and felt by some test charge q. Electric field strength: E = FE/q The unit for electric field strength equals the units force divided by units for charge: N/C Recall Coulomb’s Law: FE = k. Qq/d 2 So E = [k. Qq/d 2]/q E = k. Q/d 2 Note: Electric field strength does not depend on the small test-charge q.

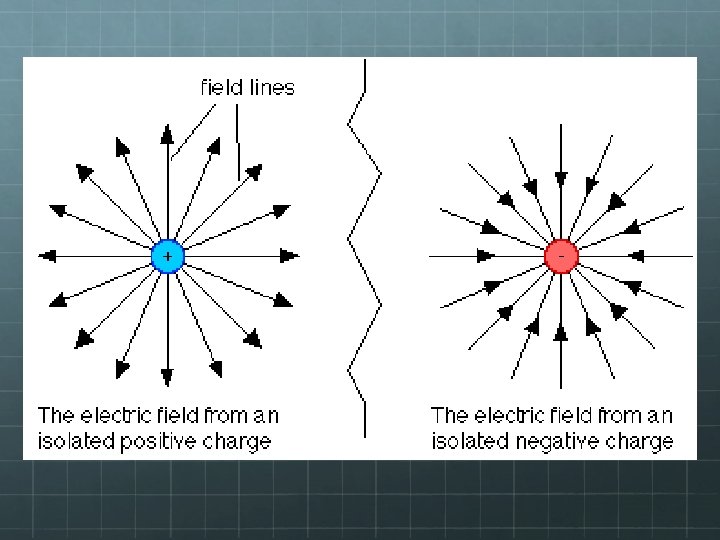
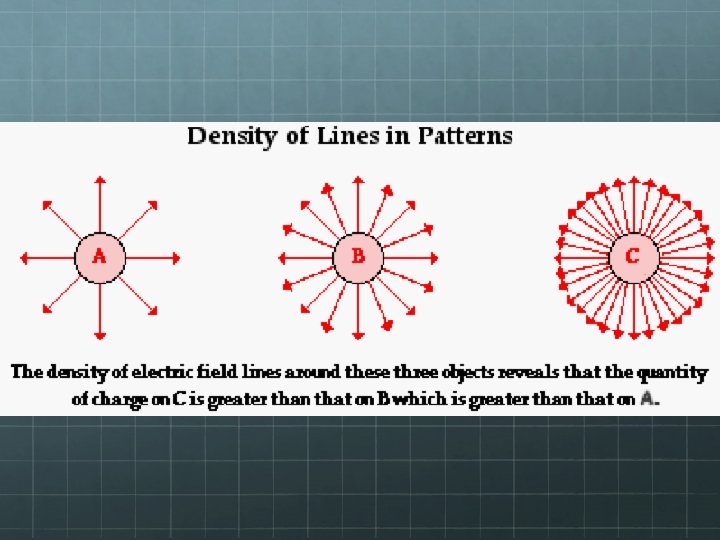
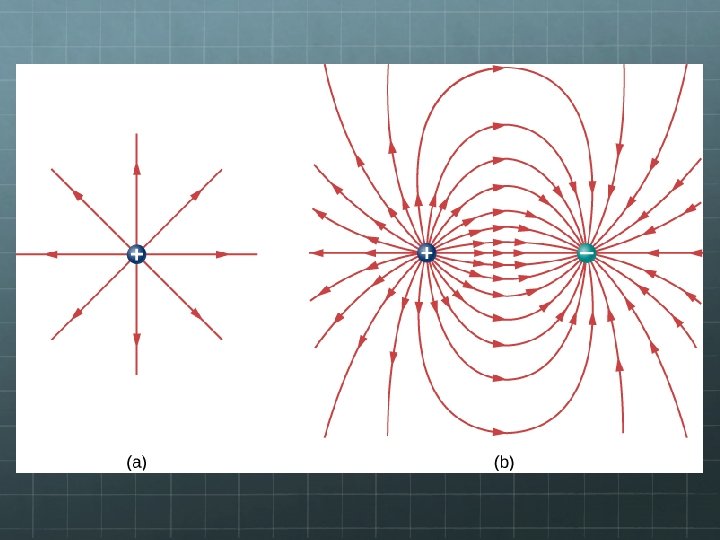
Electric Field Diagrams Arrows show the direction a small positive test charge would go if placed in the field. Electric field strength is indicated by spacing of field lines – the closer-spaced the lines, the stronger the field at that location.




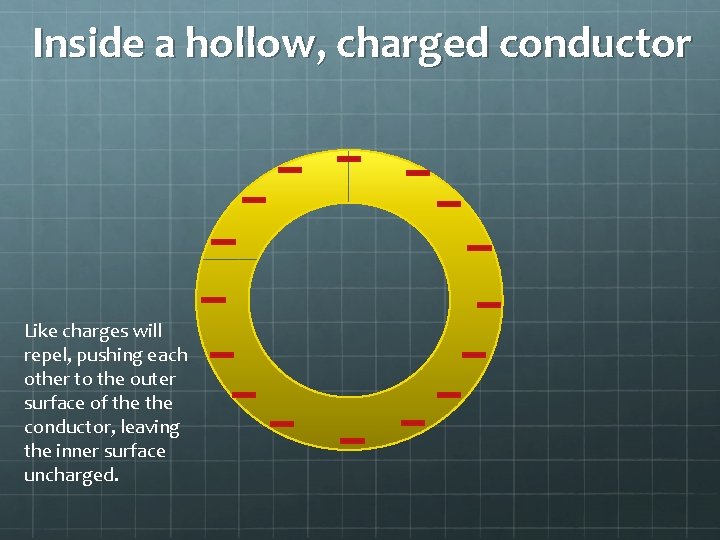
Inside a hollow, charged conductor Like charges will repel, pushing each other to the outer surface of the conductor, leaving the inner surface uncharged.

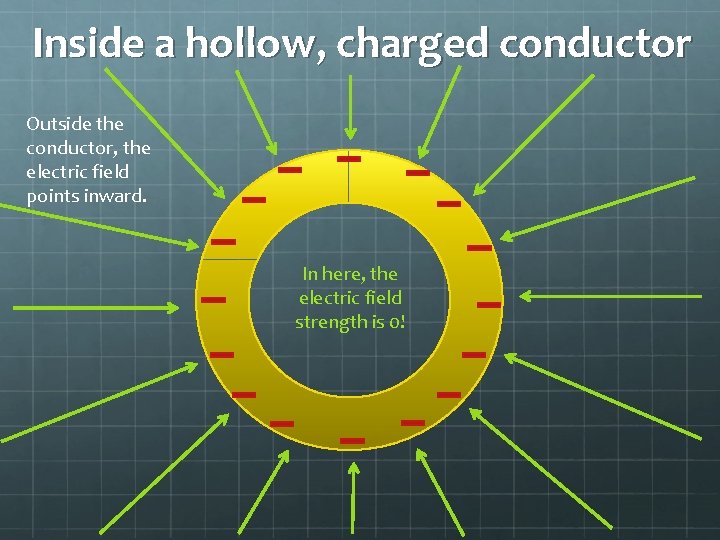
Inside a hollow, charged conductor Outside the conductor, the electric field points inward. In here, the electric field strength is 0!

So if you’re in a car struck by lightning, you’re actually safe! (From electrocution, anyway)

Work & Electric Fields It takes energy to lift mass against a gravitational field. Similarly, it takes energy to make a charge move against an electric field.

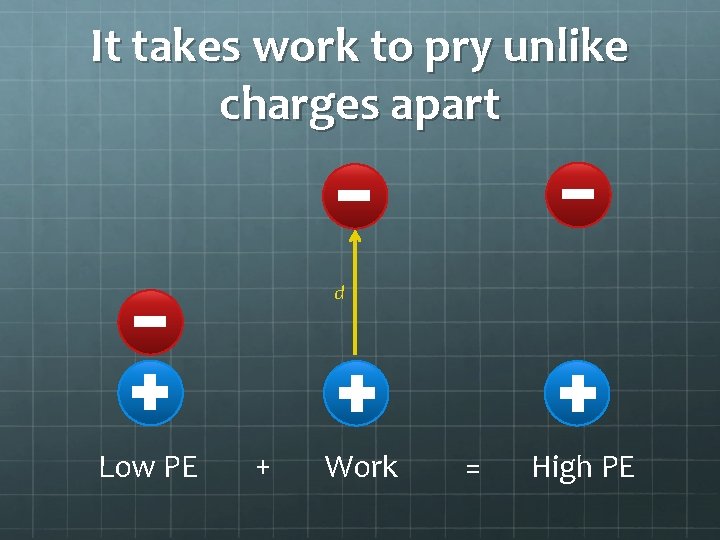
It takes work to pry unlike charges apart d Low PE + Work = High PE

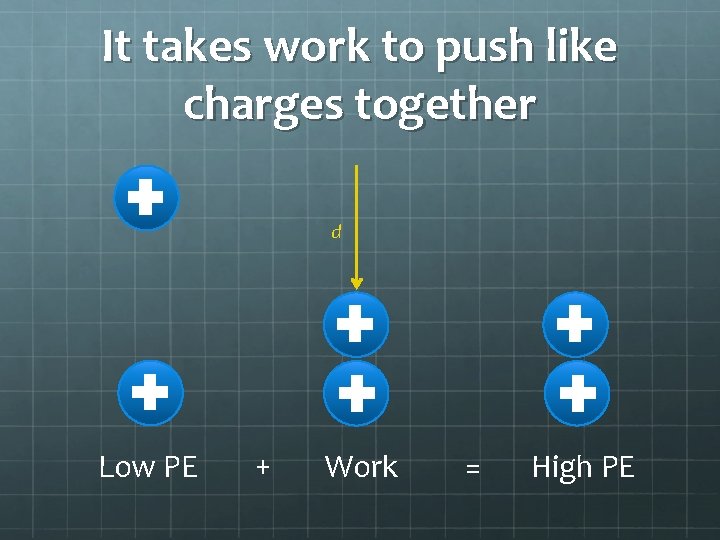
It takes work to push like charges together d Low PE + Work = High PE

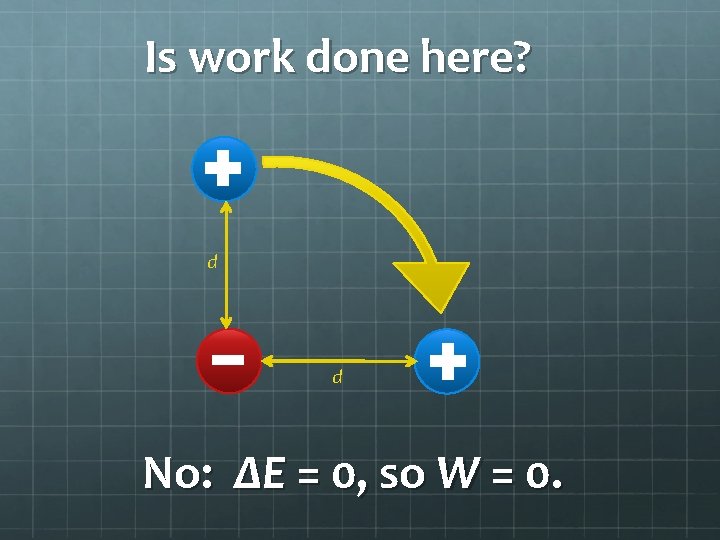
Is work done here? d d No: ΔE = 0, so W = 0.

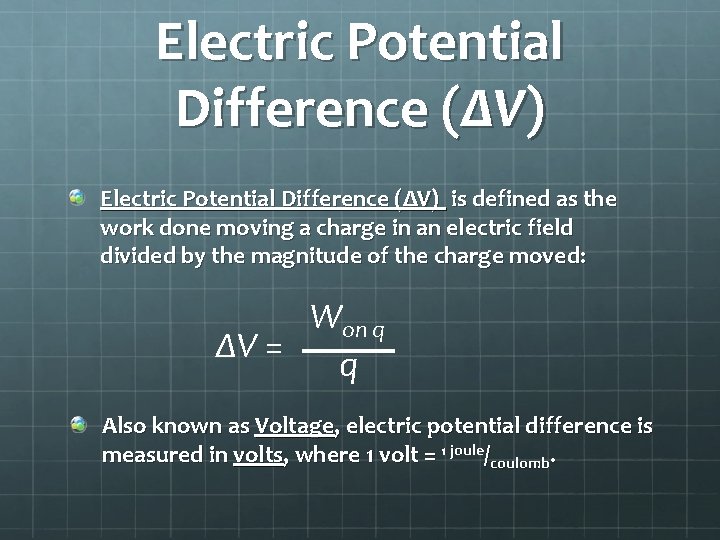
Electric Potential Difference (ΔV) is defined as the work done moving a charge in an electric field divided by the magnitude of the charge moved: Won q ΔV = q Also known as Voltage, electric potential difference is measured in volts, where 1 volt = 1 joule/coulomb.

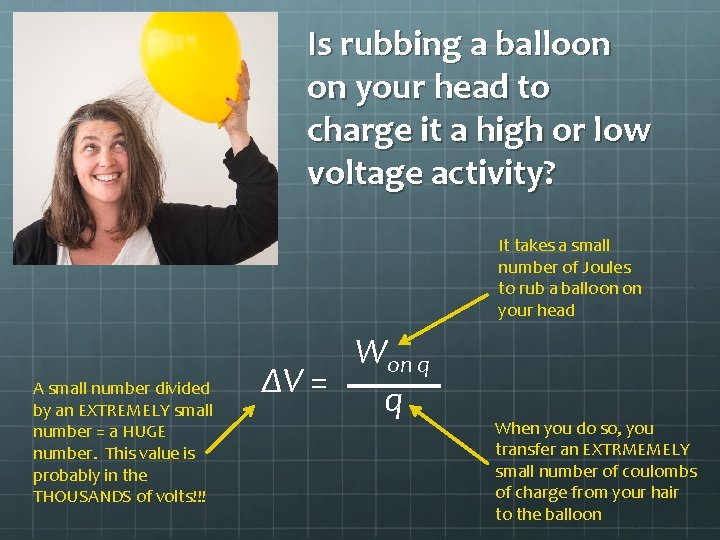
Is rubbing a balloon on your head to charge it a high or low voltage activity? It takes a small number of Joules to rub a balloon on your head A small number divided by an EXTREMELY small number = a HUGE number. This value is probably in the THOUSANDS of volts!!! Won q ΔV = q When you do so, you transfer an EXTRMEMELY small number of coulombs of charge from your hair to the balloon

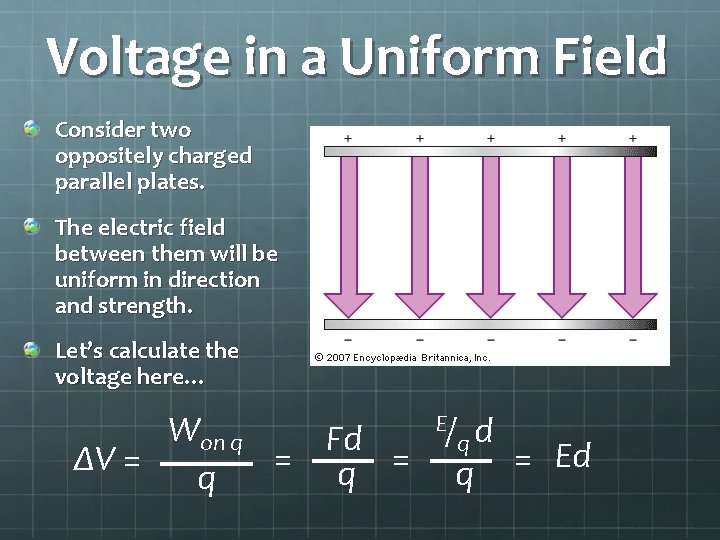
Voltage in a Uniform Field Consider two oppositely charged parallel plates. The electric field between them will be uniform in direction and strength. Let’s calculate the voltage here… Won q Fd = q = ΔV = q E/ qd q = Ed

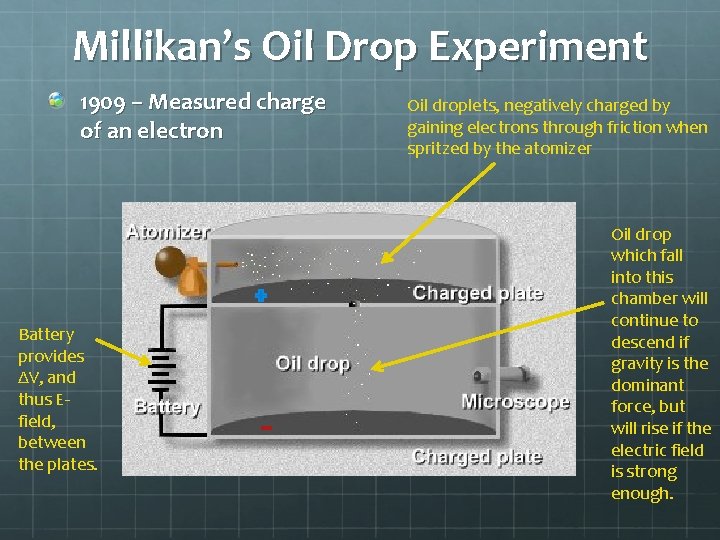
Millikan’s Oil Drop Experiment 1909 – Measured charge of an electron Battery provides ΔV, and thus Efield, between the plates. Oil droplets, negatively charged by gaining electrons through friction when spritzed by the atomizer Oil drop which fall into this chamber will continue to descend if gravity is the dominant force, but will rise if the electric field is strong enough.

Millikan adjusted voltage until he saw an oil drop floating. At this point, he knew Fg = FE. Millikan then turned off the voltage and allowed the drop to fall. The drop quickly reached terminal velocity, which Millikan measured and then used to calculate the droplet’s mass. Then, Millikan was able to express the charge of the oil drop in terms of known quantities: From earlier, FE = Fg Since E = FE/q and Fg = mg, you can say q. E = mg Solve for charge: q = mg/E

So Millikan knew the charge of the oil drop, but after the one trial, he didn’t know how many excess electrons were actually on the drop. So he repeated the experiment several more times. After several trials, he found that the oil drop always held a charge of an integer multiple of 1. 6 x 10 -19 C. Thus, he concluded, qelectron = 1. 6 x 10 -19 C.

Mass of an Electron Millikan’s results were especially important because knowing the electron’s charge allowed him to also calculate the electrons mass! Twelve years earlier, in 1897, JJ Thomson conducted an experiment at Cambridge in which he was able to calculate the charge-to-mass ratio of the electron*. Thomson didn’t know what q was, and he didn’t know what m was, but he was able to determine that q/m = 1. 759 x 1011 C/kg. Once Millikan obtained the value of q the value of m was easily obtained: m = q/(q/m) = (1. 6 x 10 -19 C)÷(1. 759 x 1011 C/kg) = 9. 1 x 10 -31 kg. *We’ll study this experiment soon when we cover magnetic fields!

Capacitance A capacitor is a device for storing electrical energy, which can then be released at will. Example application: Flashbulb on a camera. Capacitance is defined as the ratio of charge-to-voltage: C = q/ΔV. The unit for capacitance is the Farad (F). Note: 1 F = 1 joule/volt. The capacitance of a given capacitor should be constant. Thus as a capacitor is charged, it builds up a commensurate amount of voltage.

So what’s a “flux capacitor” ? ? ? My guess: Someone in Hollywood threw darts at the glossary of a physics text. Maybe it was Dogbert…