Electrical Measurement of Single Molecule Catalysis using Carbon
Electrical Measurement of Single Molecule Catalysis using Carbon Nanotubes Brett Goldsmith, Alexander Kane, Vaikunth Khalap, John Coroneus, Gregory Weiss, Phil Collins Department of Physics and Astronomy University of California Irvine
Outline Single Molecule Sensor Construction Measurement of Catalysis Reaction Rate Dynamics of the Bound State
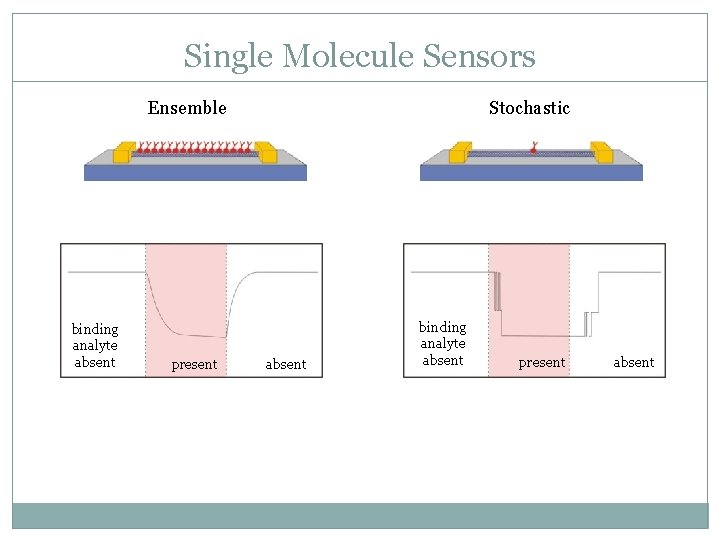
Single Molecule Sensors Ensemble binding analyte absent present Stochastic absent binding analyte absent present absent
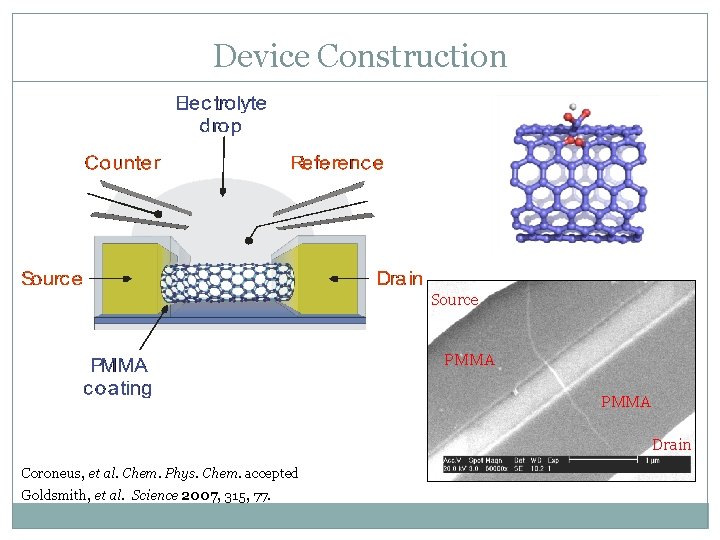
Device Construction Source PMMA Drain Coroneus, et al. Chem. Phys. Chem. accepted Goldsmith, et al. Science 2007, 315, 77.
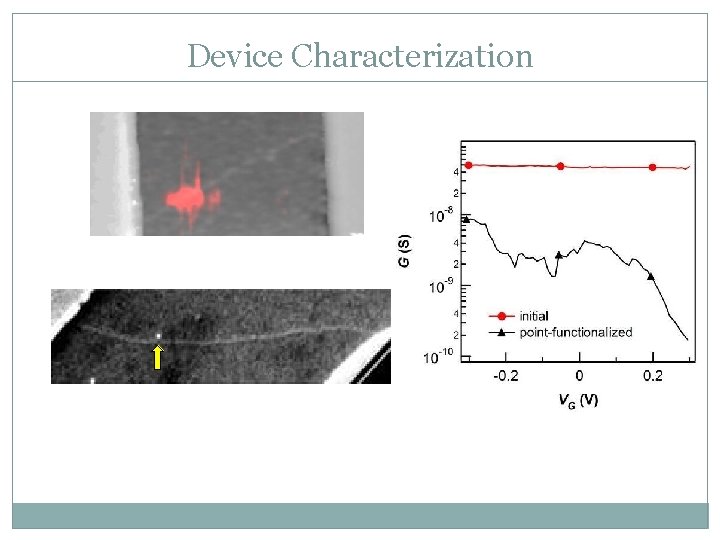
Device Characterization
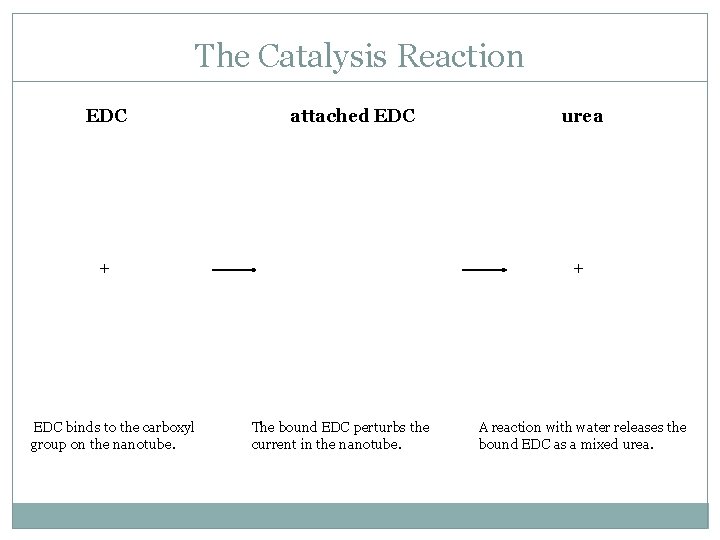
The Catalysis Reaction EDC attached EDC + EDC binds to the carboxyl group on the nanotube. urea + The bound EDC perturbs the current in the nanotube. A reaction with water releases the bound EDC as a mixed urea.
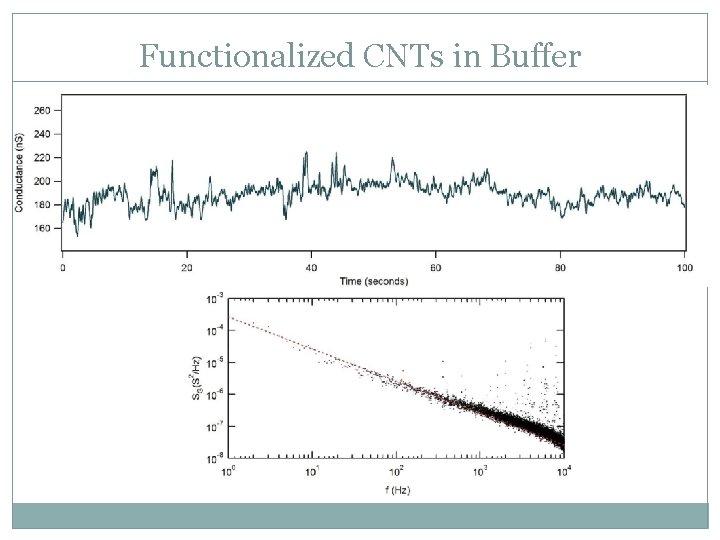
Functionalized CNTs in Buffer
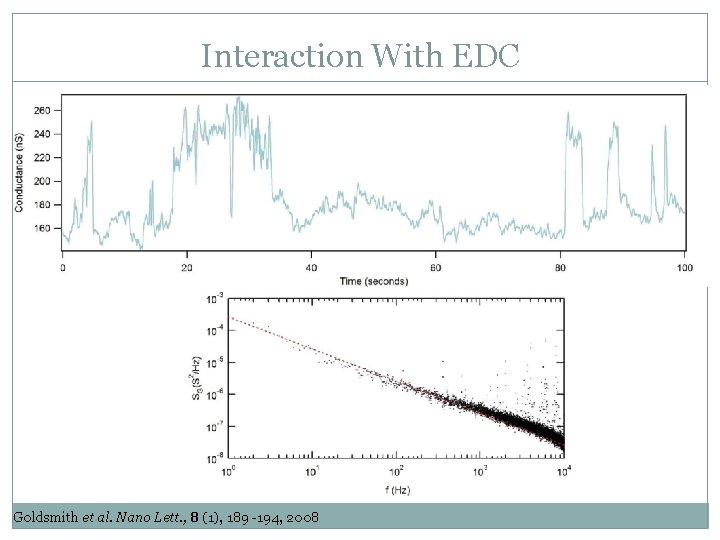
Interaction With EDC Goldsmith et al. Nano Lett. , 8 (1), 189 -194, 2008
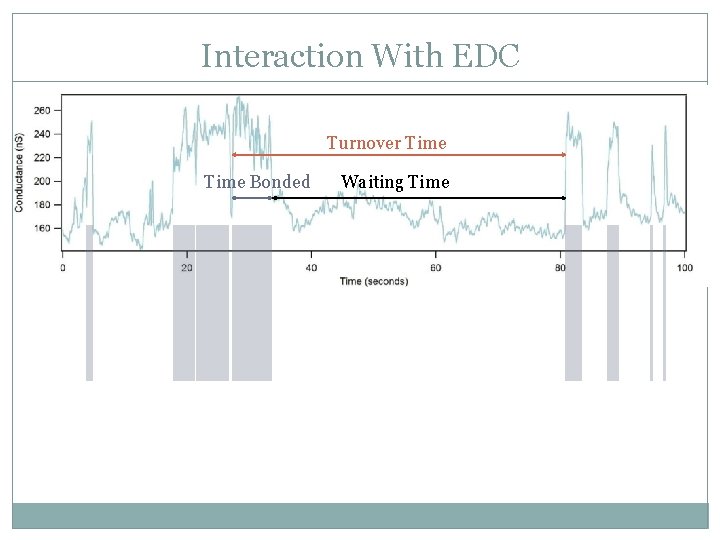
Interaction With EDC Turnover Time Bonded Waiting Time
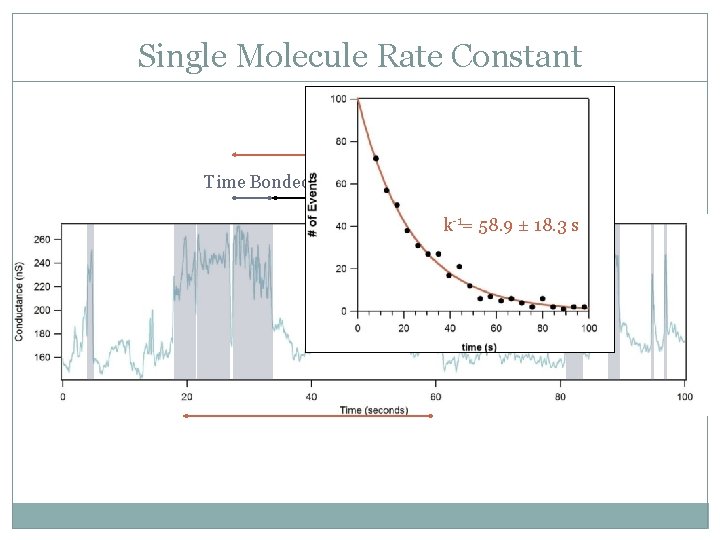
Single Molecule Rate Constant Turnover Time Bonded Waiting Time k-1= 58. 9 ± 18. 3 s
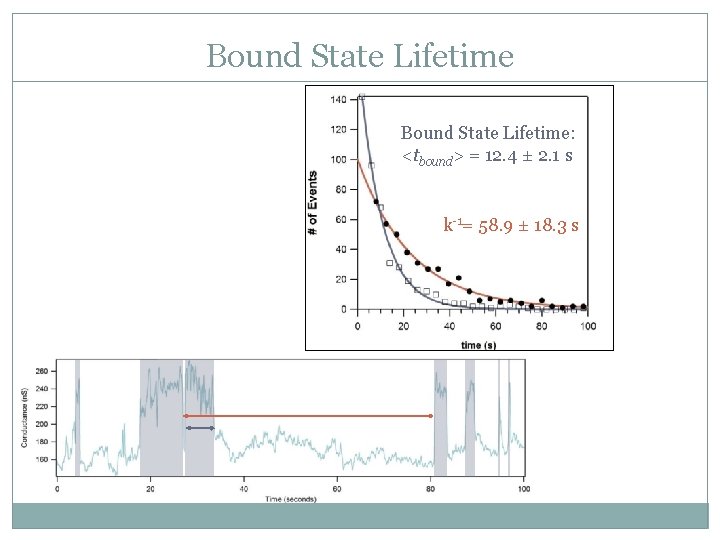
Bound State Lifetime: <tbound> = 12. 4 ± 2. 1 s k-1= 58. 9 ± 18. 3 s
Bound vs. Unbound
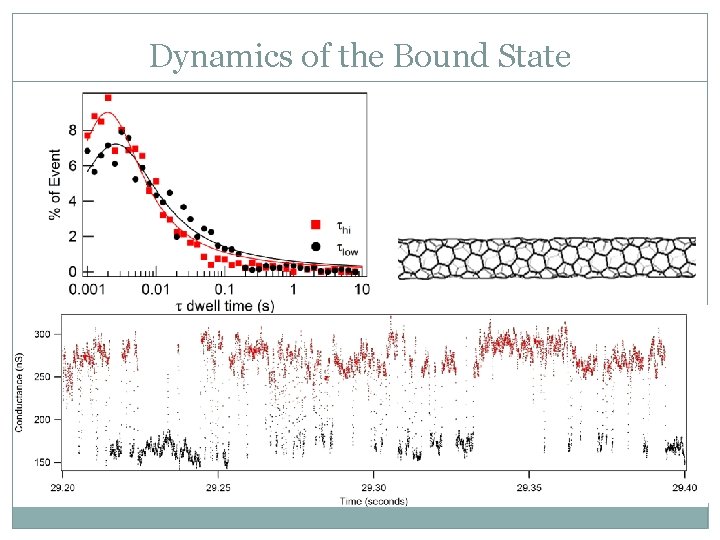
Dynamics of the Bound State
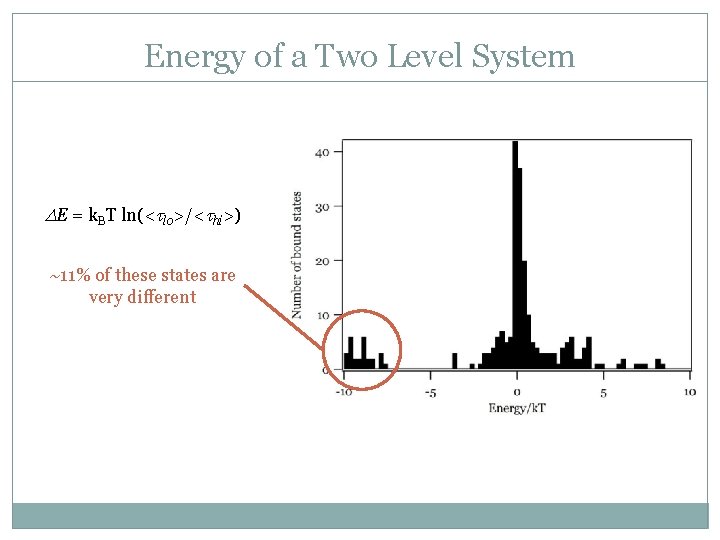
Energy of a Two Level System DE = k. BT ln(<tlo>/<thi>) ~11% of these states are very different
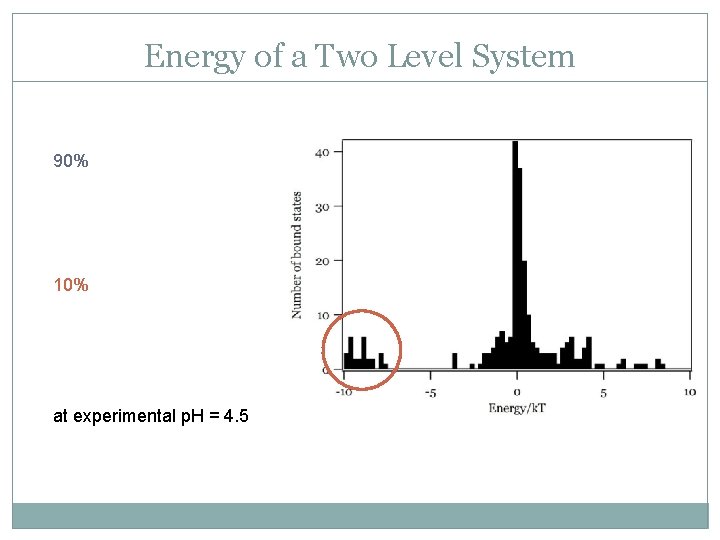
Energy of a Two Level System 90% 10% at experimental p. H = 4. 5
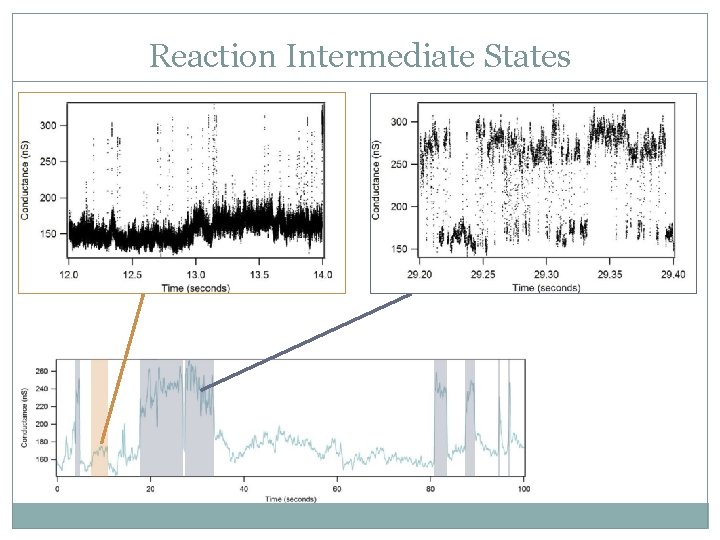
Reaction Intermediate States
Summary Goldsmith, et al. Science 2007, 315, 77. Goldsmith et al. Nano Lett. , 8 (1), 189 -194, 2008 Goldsmith et al. JMR, accepted Coroneus, et al. Chem. Phys. Lett. , accepted Dr. Phil Collins Brett Goldsmith Alex Kane Bucky Khalap Steve Hunt Danny Wan Tatyana Sheps ACS-PRF Dr. Gregory Weiss John Coroneus
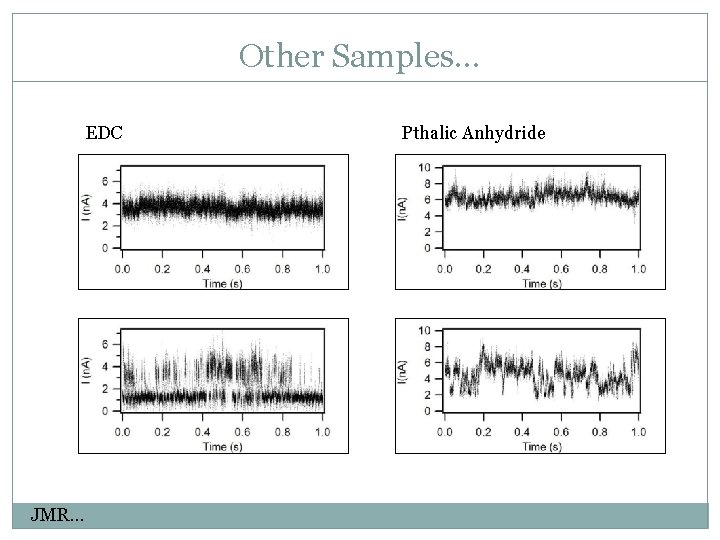
Other Samples… EDC JMR… Pthalic Anhydride
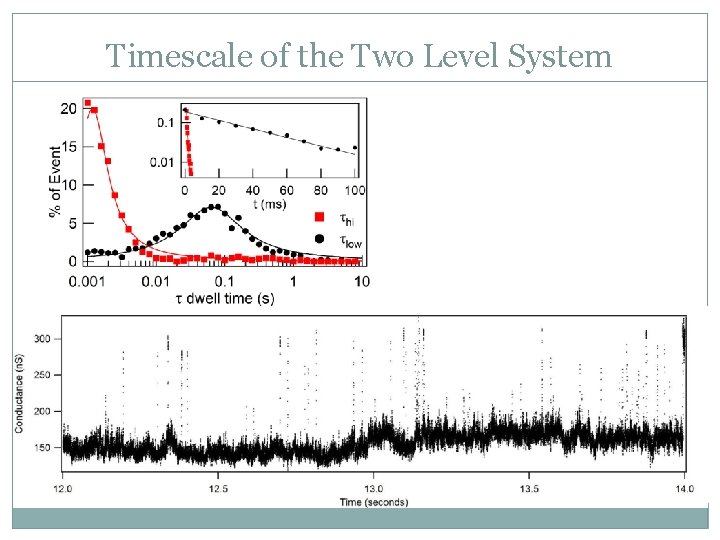
Timescale of the Two Level System
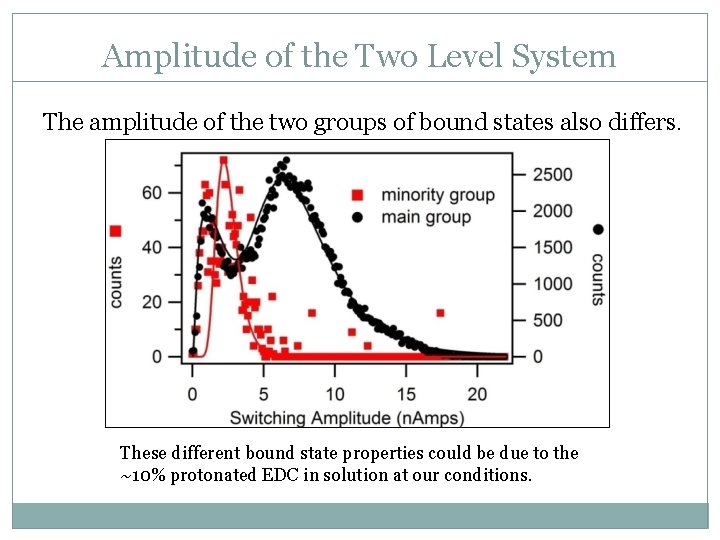
Amplitude of the Two Level System The amplitude of the two groups of bound states also differs. These different bound state properties could be due to the ~10% protonated EDC in solution at our conditions.
- Slides: 20