Electric Fields Millikans Experiment Millikan designed and used

- Slides: 16

Electric Fields & Millikan’s Experiment • Millikan designed and used an apparatus to determine the fundamental unit of charge known now as qe, the charge on an electron.

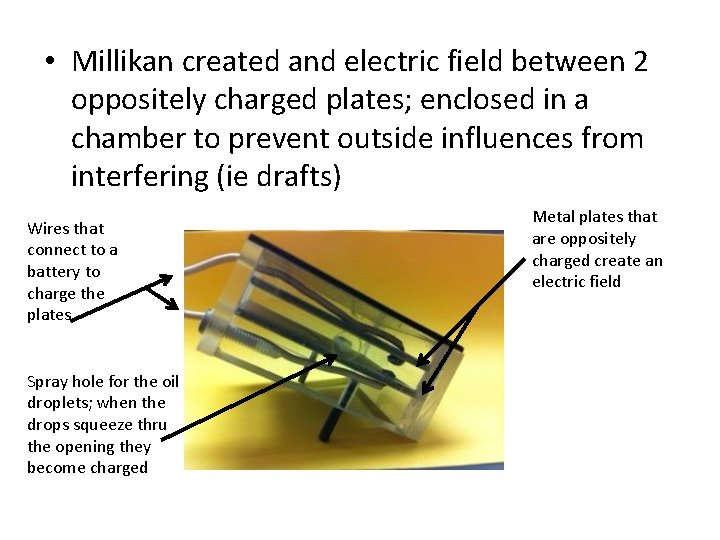
• Millikan created and electric field between 2 oppositely charged plates; enclosed in a chamber to prevent outside influences from interfering (ie drafts) Wires that connect to a battery to charge the plates Spray hole for the oil droplets; when the drops squeeze thru the opening they become charged Metal plates that are oppositely charged create an electric field

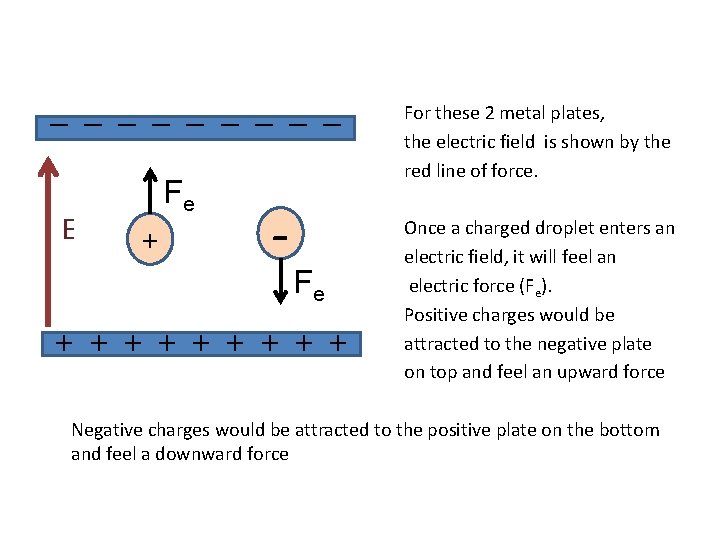
_ _ _ _ _ E Fe + - Fe + + + + + For these 2 metal plates, the electric field is shown by the red line of force. Once a charged droplet enters an electric field, it will feel an electric force (Fe). Positive charges would be attracted to the negative plate on top and feel an upward force Negative charges would be attracted to the positive plate on the bottom and feel a downward force

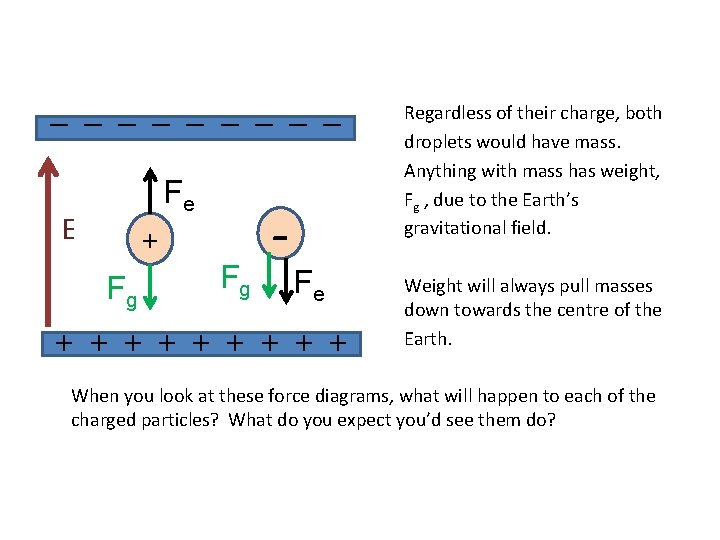
_ _ _ _ _ Fe E + Fg Fg - Fe + + + + + Regardless of their charge, both droplets would have mass. Anything with mass has weight, Fg , due to the Earth’s gravitational field. Weight will always pull masses down towards the centre of the Earth. When you look at these force diagrams, what will happen to each of the charged particles? What do you expect you’d see them do?

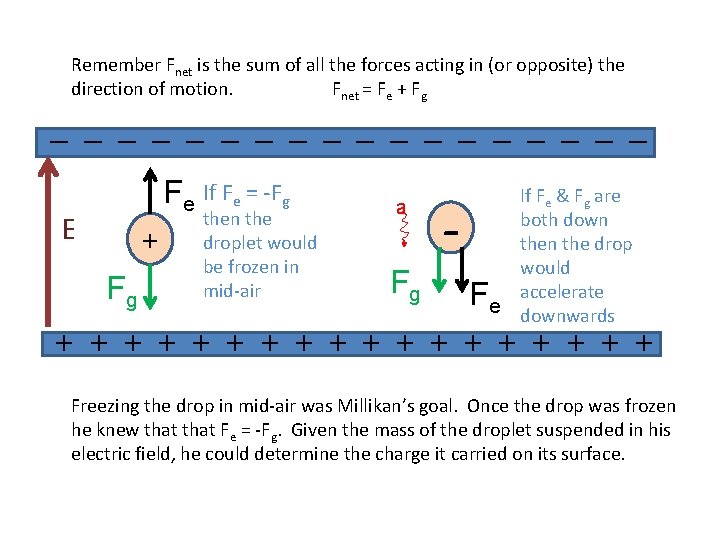
Remember Fnet is the sum of all the forces acting in (or opposite) the direction of motion. Fnet = Fe + Fg _ _ _ _ _ Fe If Fe = -Fg E + Fg then the droplet would be frozen in mid-air a Fg F If Fe & Fg are both down the drop would accelerate e downwards + + + + + Freezing the drop in mid-air was Millikan’s goal. Once the drop was frozen he knew that Fe = -Fg. Given the mass of the droplet suspended in his electric field, he could determine the charge it carried on its surface.

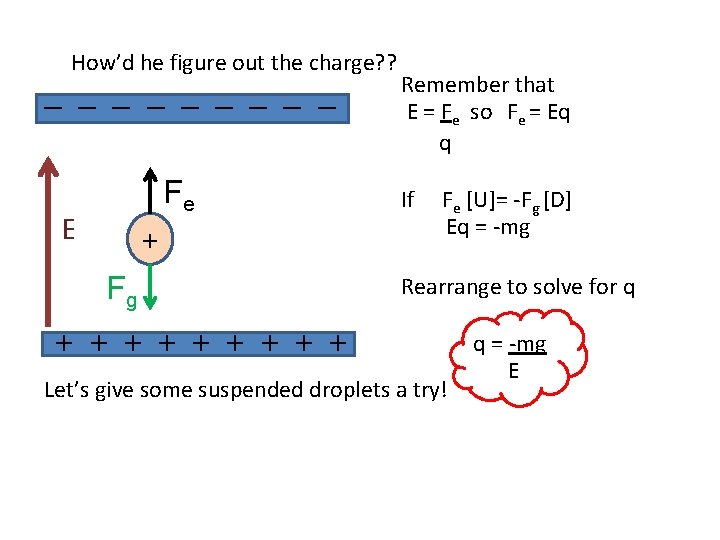
How’d he figure out the charge? ? _ _ _ _ _ Fe E + Fg Remember that E = Fe so Fe = Eq q If Fe [U]= -Fg [D] Eq = -mg Rearrange to solve for q + + + + + Let’s give some suspended droplets a try! q = -mg E

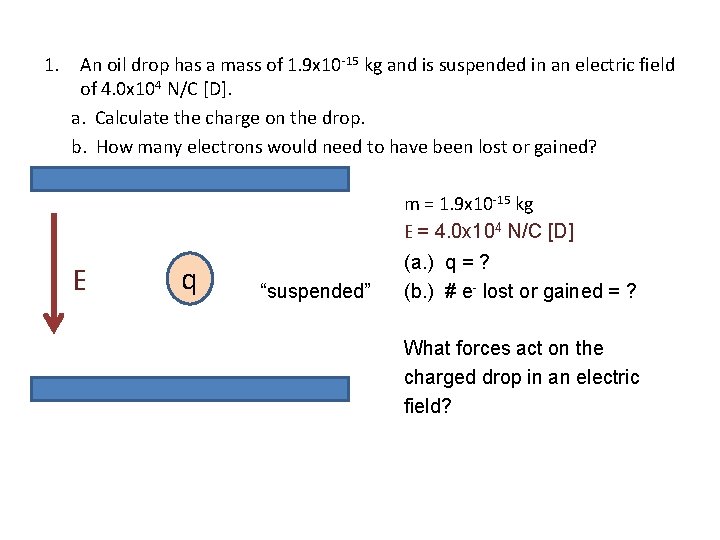
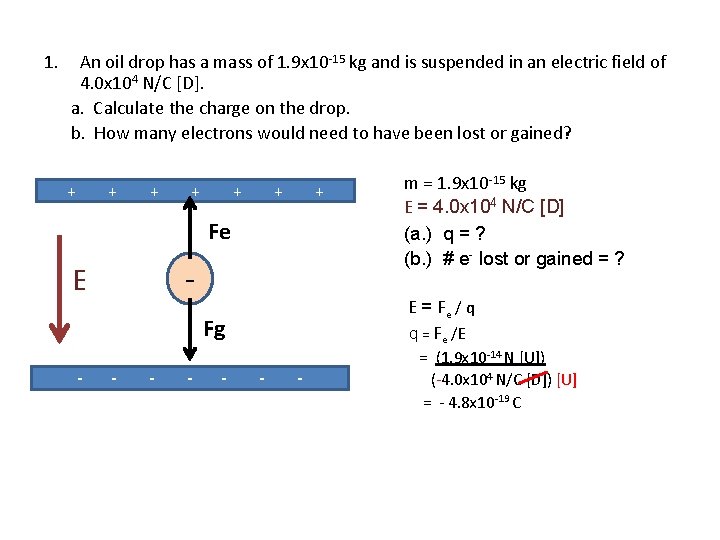
1. An oil drop has a mass of 1. 9 x 10 -15 kg and is suspended in an electric field of 4. 0 x 104 N/C [D]. a. Calculate the charge on the drop. b. How many electrons would need to have been lost or gained? m = 1. 9 x 10 -15 kg E = 4. 0 x 104 N/C [D] E q “suspended” (a. ) q = ? (b. ) # e- lost or gained = ? What forces act on the charged drop in an electric field?

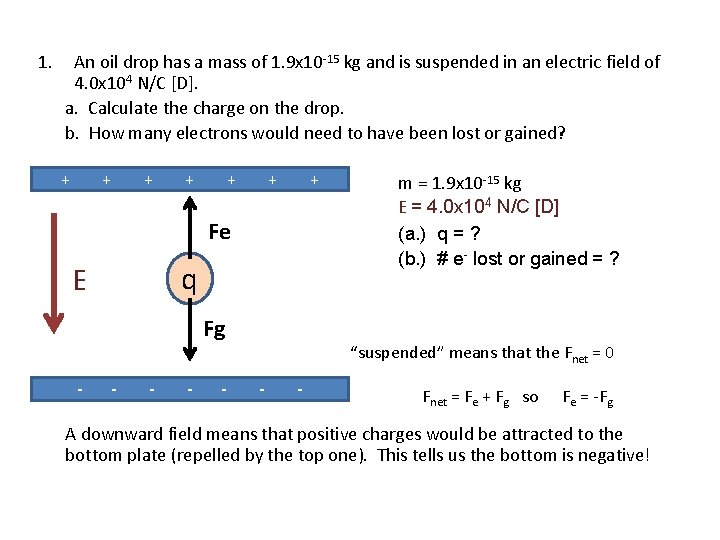
1. An oil drop has a mass of 1. 9 x 10 -15 kg and is suspended in an electric field of 4. 0 x 104 N/C [D]. a. Calculate the charge on the drop. b. How many electrons would need to have been lost or gained? + + + + Fe q E Fg - - - m = 1. 9 x 10 -15 kg E = 4. 0 x 104 N/C [D] (a. ) q = ? (b. ) # e- lost or gained = ? “suspended” means that the Fnet = 0 - - Fnet = Fe + Fg so Fe = -Fg A downward field means that positive charges would be attracted to the bottom plate (repelled by the top one). This tells us the bottom is negative!

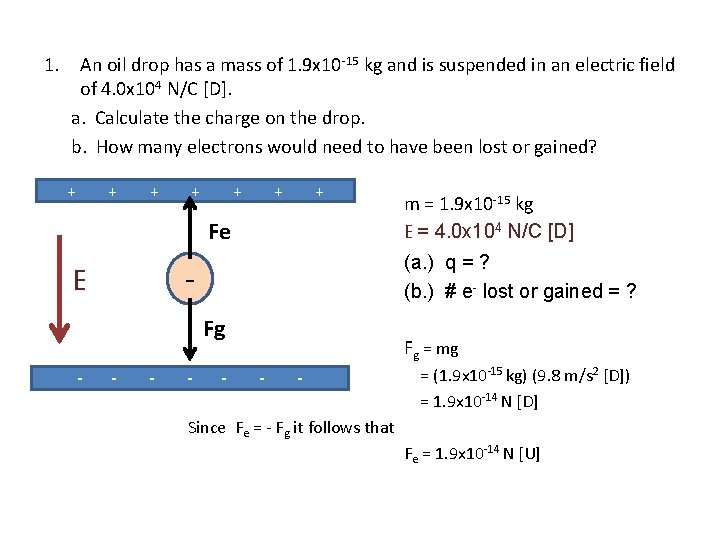
1. An oil drop has a mass of 1. 9 x 10 -15 kg and is suspended in an electric field of 4. 0 x 104 N/C [D]. a. Calculate the charge on the drop. b. How many electrons would need to have been lost or gained? + + + + Fe (a. ) q = ? (b. ) # e- lost or gained = ? - E Fg - - m = 1. 9 x 10 -15 kg E = 4. 0 x 104 N/C [D] - Fg = mg - - = (1. 9 x 10 -15 kg) (9. 8 m/s 2 [D]) = 1. 9 x 10 -14 N [D] Since Fe = - Fg it follows that Fe = 1. 9 x 10 -14 N [U]

1. An oil drop has a mass of 1. 9 x 10 -15 kg and is suspended in an electric field of 4. 0 x 104 N/C [D]. a. Calculate the charge on the drop. b. How many electrons would need to have been lost or gained? + + + + Fe - E E = Fe / q q = Fe /E Fg - - - m = 1. 9 x 10 -15 kg E = 4. 0 x 104 N/C [D] (a. ) q = ? (b. ) # e- lost or gained = ? - - = (1. 9 x 10 -14 N [U]) (-4. 0 x 104 N/C [D]) [U] = - 4. 8 x 10 -19 C

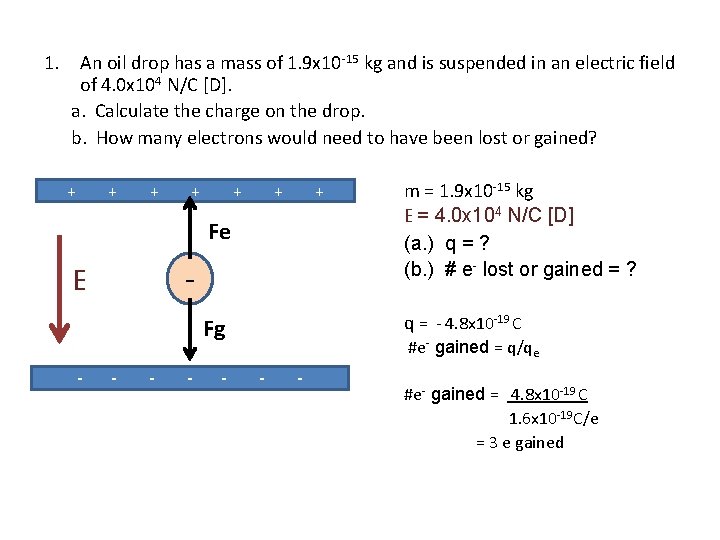
1. An oil drop has a mass of 1. 9 x 10 -15 kg and is suspended in an electric field of 4. 0 x 104 N/C [D]. a. Calculate the charge on the drop. b. How many electrons would need to have been lost or gained? + + + Fg q = - 4. 8 x 10 -19 C + + + - E - Fe m = 1. 9 x 10 -15 kg E = 4. 0 x 104 N/C [D] (a. ) q = ? (b. ) # e- lost or gained = ? + - - #e- gained = q/qe - - #e- gained = 4. 8 x 10 -19 C 1. 6 x 10 -19 C/e = 3 e gained

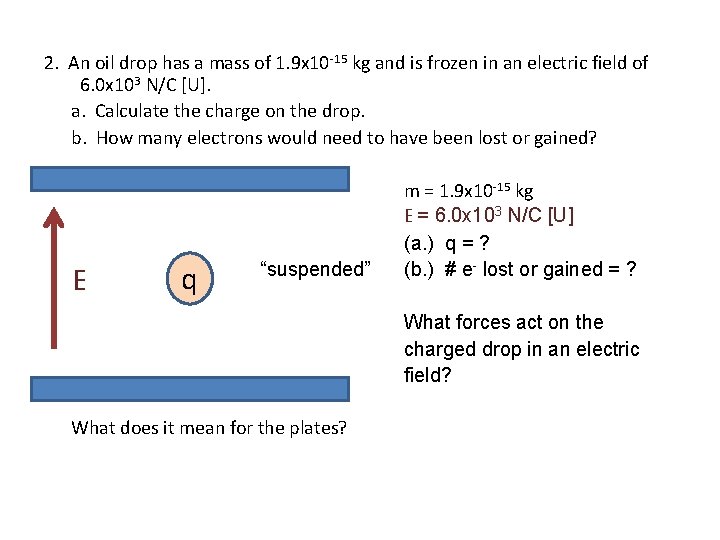
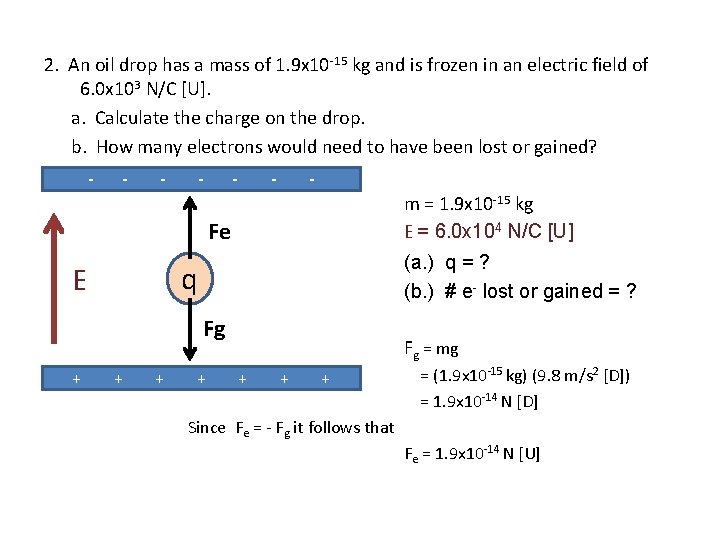
2. An oil drop has a mass of 1. 9 x 10 -15 kg and is frozen in an electric field of 6. 0 x 103 N/C [U]. a. Calculate the charge on the drop. b. How many electrons would need to have been lost or gained? E q “suspended” m = 1. 9 x 10 -15 kg E = 6. 0 x 103 N/C [U] (a. ) q = ? (b. ) # e- lost or gained = ? What forces act on the charged drop in an electric field? What does it mean for the plates?

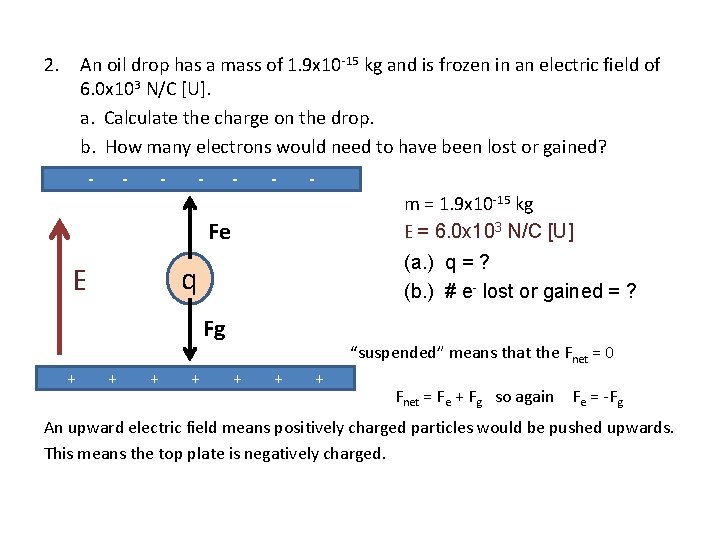
2. An oil drop has a mass of 1. 9 x 10 -15 kg and is frozen in an electric field of 6. 0 x 103 N/C [U]. a. Calculate the charge on the drop. b. How many electrons would need to have been lost or gained? - - - - m = 1. 9 x 10 -15 kg E = 6. 0 x 103 N/C [U] Fe (a. ) q = ? (b. ) # e- lost or gained = ? q E Fg + + “suspended” means that the Fnet = 0 + + + Fnet = Fe + Fg so again Fe = -Fg An upward electric field means positively charged particles would be pushed upwards. This means the top plate is negatively charged.

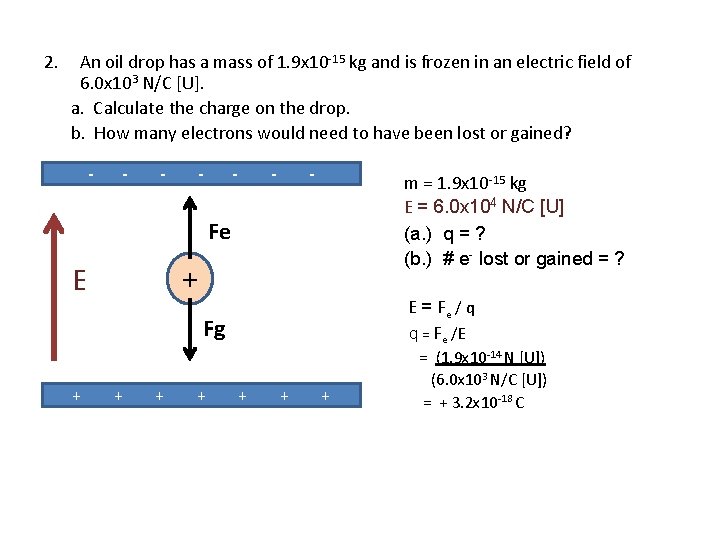
2. An oil drop has a mass of 1. 9 x 10 -15 kg and is frozen in an electric field of 6. 0 x 103 N/C [U]. a. Calculate the charge on the drop. b. How many electrons would need to have been lost or gained? - - - - m = 1. 9 x 10 -15 kg E = 6. 0 x 104 N/C [U] Fe (a. ) q = ? (b. ) # e- lost or gained = ? q E Fg + + Fg = mg + + + = (1. 9 x 10 -15 kg) (9. 8 m/s 2 [D]) = 1. 9 x 10 -14 N [D] Since Fe = - Fg it follows that Fe = 1. 9 x 10 -14 N [U]

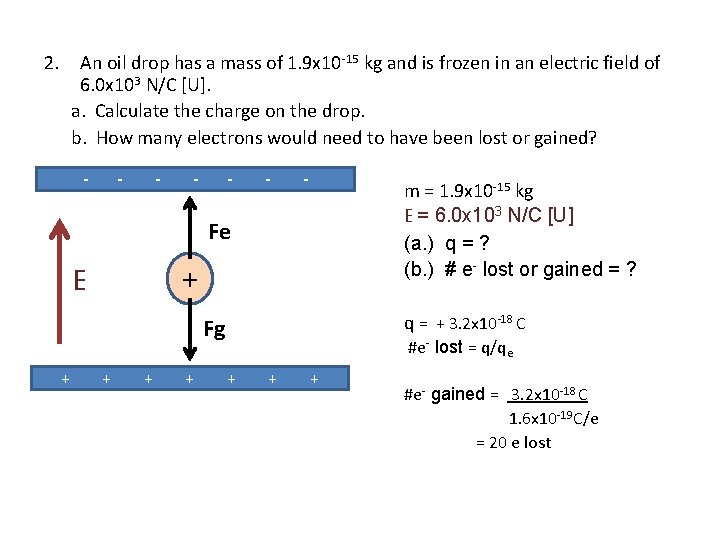
2. An oil drop has a mass of 1. 9 x 10 -15 kg and is frozen in an electric field of 6. 0 x 103 N/C [U]. a. Calculate the charge on the drop. b. How many electrons would need to have been lost or gained? - - - - m = 1. 9 x 10 -15 kg E = 6. 0 x 104 N/C [U] (a. ) q = ? (b. ) # e- lost or gained = ? Fe + E E = Fe / q q = Fe /E Fg + + + + = (1. 9 x 10 -14 N [U]) (6. 0 x 103 N/C [U]) = + 3. 2 x 10 -18 C

2. An oil drop has a mass of 1. 9 x 10 -15 kg and is frozen in an electric field of 6. 0 x 103 N/C [U]. a. Calculate the charge on the drop. b. How many electrons would need to have been lost or gained? - - - Fe m = 1. 9 x 10 -15 kg E = 6. 0 x 103 N/C [U] (a. ) q = ? (b. ) # e- lost or gained = ? Fg q = + 3. 2 x 10 -18 C + E + - + + + #e- lost = q/qe + + + #e- gained = 3. 2 x 10 -18 C 1. 6 x 10 -19 C/e = 20 e lost