Electric Charge and Static Electricity Page 30 Essential
















- Slides: 25

Electric Charge and Static Electricity Page 30

Essential questions l 1. What is the connection between parts of an atom and charged particles? l 2. What characteristics define electric currents?


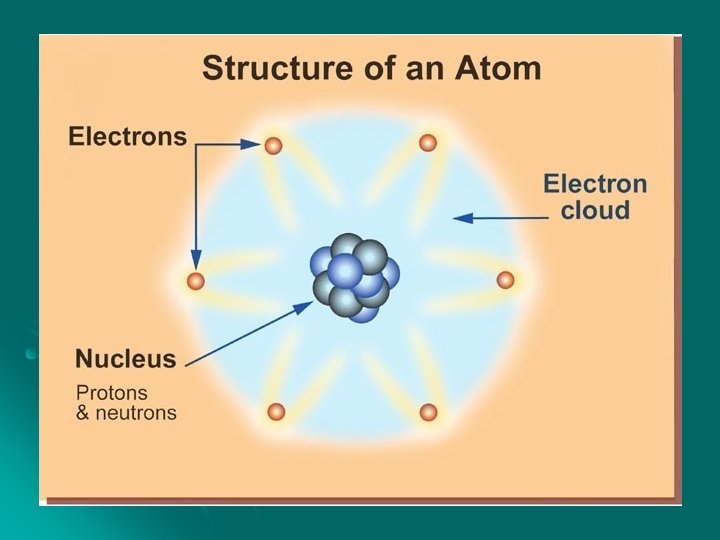
ATOMS and charge l Atoms ARE MADE OF PARTICLES: l Protons are LOCATED ____ l Protons have a ______ charge l Neutrons are located _______ l Neutrons have a _____ charge l Electrons are located _______ l Electrons have a _____ charge

Review time Electrons are found inside the nucleus. True or false

Correct answer is false

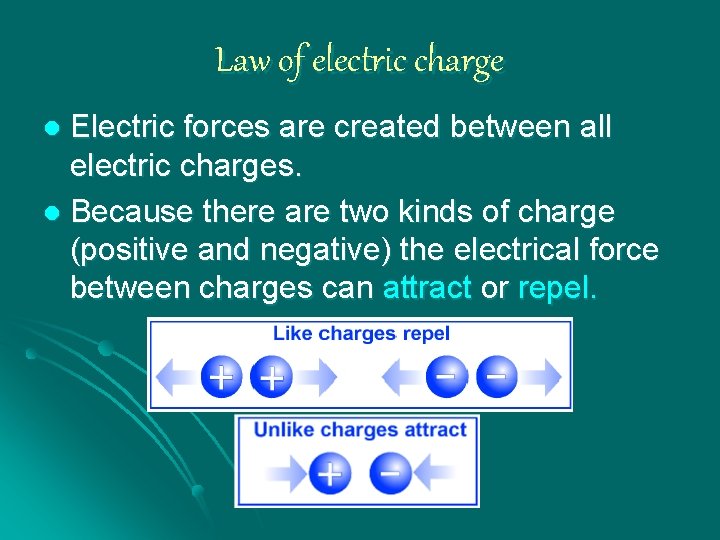
Law of electric charge Electric forces are created between all electric charges. l Because there are two kinds of charge (positive and negative) the electrical force between charges can attract or repel. l

Electric force l l Is the force between charged objects. Strength is determined by 2 factors: l The size of the charges. l Distance between charges

Electric field l l l Is a region around a charged particle that can exert a force on another charged particle. Objects become charged because the atoms in the objects can gain or lose electrons. If the atoms lose electrons the object becomes (+). If the atoms gain electrons the object becomes (-).

How objects become charged l l l Friction-rubbing of 2 objects together Conduction- occurs when electrons are transferred from 1 object to another by direct contact. Induction- occurs when an uncharged object are rearranged without direct contact with a charged object.

Conservation of charge l l l when you charge objects by any method, no charges are created or destroyed. Electrons simply move from 1 atom to another. This produces objects or regions with different charges. Since charges are not created or destroyed, charge is said to be conserved.

Detecting charge l To determine charge use an electroscope.

Moving charges 2 groups- conductors & insulators. l Conductor- is a material in which charges can move easily. Mainly metals. l Examples- copper, silver, aluminum, and mercury l Water is a conductor l

insulators Insulator- is a material in which charges cannot move easily. l Electrons are tightly bound to atoms. l Examples- plastic, rubber, glass, wood, and air. l

Static electricity S. e. – is the buildup of electricity charges on an object. l If an object is static it is not moving. l Electric discharge – the loss of static electricity. l

lightning l l Example of electric discharge Figure 6 page 479

LIGHTNING Lighting rod- is a pointed rod connected to the ground by a wire. l Lightning rods are “grounded” meaning they are in contact with earth. l Earth absorbs charge so no damage is done to buildings. l

Things to remember l Charge is a physical property. Objects with a (+) or (-) charge exert a force on other charged objects l l Lightning and the shock you receive from a doorknob are example of electric discharge. What is a conductor/ insulator?

Electrical energy Section 2

l l l Electrical energy- the energy of electric charges. Electric current- flow of electrical charges. One common way to produce electric current is through chemical reactions in a battery. l l Cell- is a device that produces an electrical current by converting chemical energy to electrical energy. Battery- converts chemical energy into electrical energy and is made of several cells.

Parts of a cell l Every cell contains a mixture of chemicals that conducts a current. l l The mixture is called an electrolyte. Chemical reactions in the electrolyte convert chemical energy into electrical energy.

Parts…. . l Electrode- is the part of a cell through which charges enter or exit.

Cell types Cell are divided into 2 groups. l Wet cells- contain liquid electrolytes. (example car battery) l Dry cells- contain electrolytes that are solids or paste-like. (ex. Batteries) l

Terms Potential difference- energy per unit charge; specifically, the difference in energy per unit charge as a charge moves between 2 points in an electric circuit. l Measured in volts l

l Photocell- is the part of a solar panel that converts light into electrical energy. l Thermocouples- used to monitor the temperature of car engines, furnaces, and ovens.